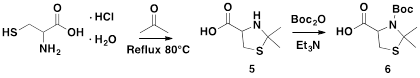58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR752090-UR7: Novel Polymer Coupling via Thiazolidine Chemistry
Philip J. Costanzo, PhD, California Polytechnic State University
Thiazolidine chemistry is a commonly used reaction used in biological systems because the reaction requires the presence of both cysteine (a common amino acid) and an aldehyde or ketone. If cysteine residues could be incorporated into a polymer then a variety of applications could be developed. For these reasons the attachment of cysteine residues in a facile way via reversible addition fragmentation chain transfer (RAFT) polymerization or small molecule synthesis was researched. The incorporation of latent cysteine residues into the polymer via post polymerization modification proved to be less successful. However protected cysteine molecules have been successfully ligated onto polymerizable monomers and have been shown to be easily deprotected in the presence of an acid source.
Part 1 - Post Polymerization method
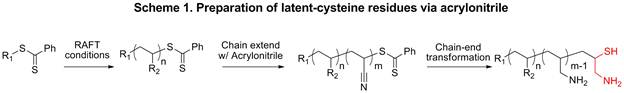
Scheme 1 illustrates the overall methodology to incorporate cysteine-like residues via a post-polymerization modification method. A prepolymer is prepared followed by chain extension with acrylonitrile followed by chain end transformation to yield the desired functionality.
Scheme 2. Synthesis of RAFT transfer agents.
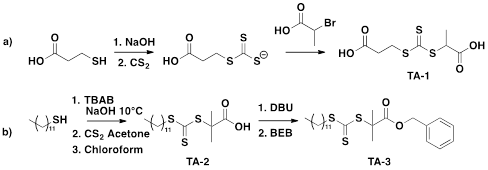
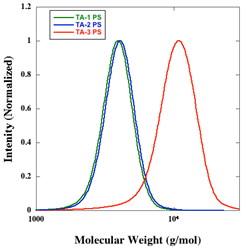
The synthesis of new transfer agents was carried out to find a simple and efficient synthesis that did not require much purification and afforded high yields. Homopolymerization of styrene for all the transfer agents was straightforward and proceeded with high conversion (>80%). In the presence of the RAFT transfer agent, the polymerization styrene was able to proceed in a controlled fashion as all transfer agents had a polydispersity index (PDI) of less than 1.15 (Figure 1).
Figure 1. GPC trace of the homopolymerization of styrene with three transfer agents.
A small block of acrylonitrile (~2 to 6 repeat units was chain extended). As previous research had shown, larger acrylonitrile blocks proved problematic. After reduction, the polymer became too hydrophilic and proved to be difficult to isolate. Additionally, the presence of extra amines compromises the efficiency of the thiazolidine coupling. As done previously, a high concentration of acrylonitrile (AN) and a relatively high concentration of AIBN (TA:AIBN 1.0:1.9) was implored. All prepolymers displayed an ideal chain extension as there is a shift in molecular weight in both the low molecular weight and high molecular regions.
Following the chain extension of AN, reduction conditions were utilized to expose the cysteine-like functionality. MTS and propylamine were added to cleave the trithiocarbonates and protect the exposed thiol simultaneously. The propylamine reacts with the trithiocarbonates end group and cleaves thiocarbonylthio producing a free thiol. Because the reaction is done in the presence of oxygen, the thiols are readily oxidized and can form disulfide bonds with the excess MTS in the reaction. After the cleavage of the transfer agent and the subsequent protection with MTS, the polyacrylonitrile block was reduced to a polyallylamine block. This was done through the addition of tetrabutyl ammonium borohydride (TBAB) in an ethanol:THF mixture. The mixture effervesced initially and changed from a white solution to a deep brown over time.
Lastly, coupling experiments of all three polymers prepared from their respective transfer agent was performed. For each reaction, dithiothreitol (DTT) was added to cleave the disulfide bond that was made with between MTS and the polymer. After the reaction had stirred for 24 hours, a half molar equivalent of isophthalaldehyde was added to the reaction and allowed to stir for an additional 24 hours. None of the prepared prepolymers displayed complete coupling. In an effort to improve coupling efficiency, coupling conditions were optimized including the presence of base (potassium carbonate), the amount dithiothreitol, time, and solvent selection were analyzed to see if a maximum efficiency could be obtained.
While initial results showed that all transfer agents did not eventually achieve 100% coupling, a re-examination of the conditions showed that coupling efficiency could be manipulated. It was learned that increasing the polarity of the solvent, increasing the amount of DTT, and the addition of K2CO3 all help to increase the efficiency of thiazolidine ring formation. Allowing the ring to form at longer periods of time did not correlate to any significant increase in ring formation.
A limitation to this method is that only the chain ends of the polymer can provide the latent cysteine residues necessary to achieve coupling. Additionally, multiple post-polymerization modifications were necessary and eventually effected recovery of the polymer.
Part II - Protected cysteine method
After determining the limitations of the post-polymerization methods, a secondary method based upon a protected cysteine was developed, Scheme 3.
Scheme 3 Synthesis of protected cysteine.
The synthesis of 5 was simple and efficient. L-cysteine HCl does not appear soluble in acetone, but when refluxed at 80 °C, the solution eventually becomes homogeneous and 5 precipitates out. It was found that residual L-cysteine HCl could be avoided by filling the reaction vessel to about three fourths full as it prevents the salt from plating out of the solvent. The synthesis of 6 proved to be exceedingly difficult. A variety of reaction conditions (6a-6e) were attempted. Two different non-nucleophilic bases triethylamine (Et3N) and N,N-diisopropylethylamine (DIPEA) were used as well a variety of solvents THF, ACN, and DMF and temperatures (r.t., 40 °C and 70 °C). The Boc protecting group is very sensitive to acid so it is possible that when HCl was added that it hydrolyzed the Boc group and reformed 5 which is readily soluble in water. A new protecting group was necessary to improve the solubility of the protected cysteine in organic solvents. A mixed anhydride method was employed, Scheme 4.
Scheme 4. Formylation of protected cysteine.
The synthesis of 11 was confirmed directly via 1H NMR after it was recrystallized from methanol and water. Current and future work is exploring the functionalization of materials using the residual carboxylic acid as a chemical handle.
Copyright © 2014 American Chemical Society