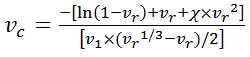58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1052308-DNI10: Graphene-Integrated Durable Rubber Sealants for Petroleum Exploration
Jingjing Qiu, PHD, Texas Tech University
1. Experimental
The graphene was produced by the modified Brodie's method [1]. 10 g of graphite was mixed with 85 g sodium chlorate and 160 ml of fuming nitric acid and followed by stirring for 24 hrs. After rinsing, the powder was obtained and tip-sonicated in ammonia solution. After air-dry, the graphene oxide (GO) powder was obtained. The GO/ammonia solution was further mixed and stirred with hydrazine to obtain reduced graphene oxide (RGO).
The solubility tests for both fluoroelastomer (FKM) and GO (or RGO) within different solvents (such as DMF, DMSO, etc) were carried out [2] and acetonitrile demonstrated excellent solubility for both FKM and GO (or RGO). FKM and GO (or RGO) were dispersed into acetonitrile respectively and were tip-sonicated for 1 hr followed by strong stirring for 3 days. The FKM/GO (or RGO) mixture was achieved using the co-coagulation method. Briefly, the FKM suspension was slowly poured into the GO (or RGO) suspension. After stirring for 4 hrs, the mixture was co-coagulated by adding large amount of de-ionized (DI) water and oven-dried after filtration. Peroxide was then mixed into the samples at 80 rpm at 80°C and further mixed in a Haake Polylab rheometer mixer to remove liquid and bubble residues. As-synthesized composites were cured by hot press at 177°C, 5 MPa for 7 min, and then post-cured at 235°C for 24 hrs in a compression mold according to ASTM D3182.
2. Characterization
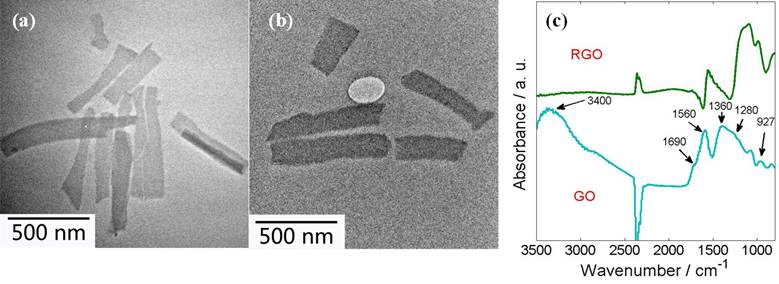
As-synthesized GO and RGO were characterized by a HITACHI T8100 Transmission Electron Microscopy (TEM) and a Nicolet iS10 Fourier-Transform Infrared (FT-IR) spectrometer. As shown in Fig. 1, the GO and RGO were mostly single-layer graphene. In the FT-IR spectra, epoxy groups, carboxyl groups, and aromatics were observed in the as-prepared GO.
Fig. 1. TEM image of the GO (a) and RGO (b); FT-IR spectra of GO and RGO (c).
The liquid-barrier properties were calculated [3-5] and summarized in Table 1. The samples were soaked in the solvent and were fetched out at specific time intervals, weighed and then returned to the solvent. The mole percent uptake Qt (sorption) of solvent was determined by the mass ratio between the absorbed solvent after t min of immersion and the rubber samples [6-8]. The crosslinking densities were measured by the swelling tests in methyl ethyl ketone (MEK) according to ASTM D6814. The composite samples were taken out and weighed after soaking for 72 hrs. After oven-dry, the weights were measured again, as shown in the Flory-Rehner equation [9-11]:
where v1 is the molecular volume of solvent, vr is the volume fraction of the rubber composite in the swollen gel, and c is Flory-Huggins polymer solvent interaction parameter.
The crosslink density was increased by 20.8 % for GO/FKM nanocomposites, indicating the crosslinking between GO and FKM after vulcanization. The diffusion and permeation coefficients were reduced with the addition of GO or RGO due to the reduced available free space within matrix and restricted segmental mobility of the rubber matrix.
Table 1. The crosslink density and liquid barrier properties of FKM nanocomposites
Sample | Crosslink density / mol/ cm3 x10-5 | Diffusion Coefficient / m2/s x10-6 | Sorption Coefficient / g/g | Permeability Coefficient/ m2/s x10-5 |
Control | 9.1 | 20.6 | 4.02 | 8.28 |
2% GO/FKM | 11.0 | 15.5 | 3.83 | 5.94 |
2% RGO/FKM | 8.3 | 19.2 | 3.85 | 7.41 |
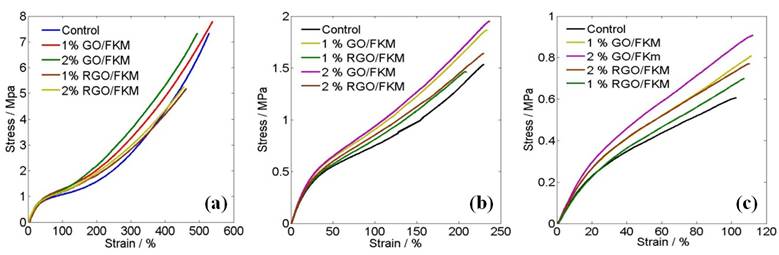
The tensile tests were performed on a SHIMADZU Universal Tester at 25°C, 75°C and 150°C in a Hamilton temperature control chamber according to ASTM D412. As shown in Fig.2, the GO/FKM nanocomposites indicated highest strength and toughness due to their higher crosslinking density and the strong interfacial bonding. At elevated temperatures, GO/FKM nanocomposites demonstrated better thermal stability for both tensile strength and toughness.
Fig. 2. Tensile tests of the FKM nanocomposites at 25 oC (a), 75 oC (b) and 150 oC (c)
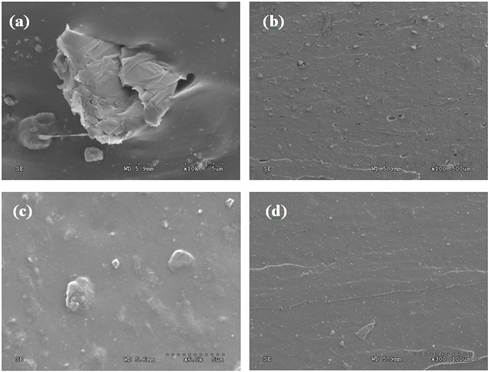
The morphology of the rupture cross-section was investigated by a HITACHI S4300 Scanning Electron Microscopy (SEM), as shown in Fig. 3. It indicated that GO was well dispersed in FKM matrix with strong interface, while RGO is weakly bonded with FKM matrix.
Fig. 3. SEM images for the 2% RGO/FKM samples (a), (b) and the 2% GO/FKM samples (c), (d).
3. Results and Discussion
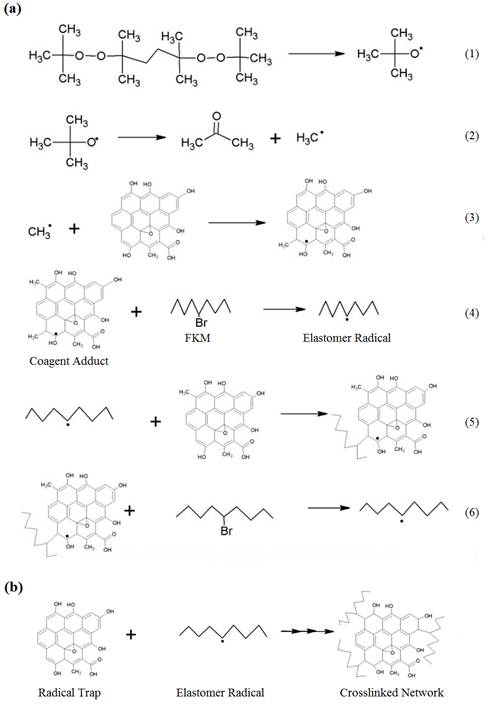
The reaction scheme is illustrated in Fig. 4 and was confirmed by Br concentration according to EDAX [12-13] and differential scanning calorimetric (DSC) tests. During vulcanization, the ally alcohols on the GO react with peroxide free radicals to provide initial free radicals and further activate more GO to be involved in the crosslinking reaction. The free radicals abstract bromine from FKM chain or transfer to other GO to provide more stable radicals.
Fig. 4. (a) The reaction steps and (b) the summarized crosslinking reaction scheme.
4. Impact of the Research
The research will impact the further development and applications of graphene in petroleum exploration materials. (1) A cost-effective processing method using ultrosonication and co-coagulation technique was employed to fabricate graphene-integrated FKM nanocomposites. (2) The effects of processing, surface modification, graphene fractions, and curing agent on the crosslinking network structure and properties of FKM nanocomposites were revealed and will be further optimized by material/process design. (3) A novel graphene/FKM nanocomposite with superior high-temperature mechanical properties, and gas-/liquid-barrier properties is anticipated to bring a breakthrough in next-generation gas/liquid sealant materials that are able to stand up in harsh environments for petroleum exploration. The PI will develop subsequent research proposals about nanomaterials enhanced elastomers and hydrogels. The results will also advance the PI's career path by dissemination through annual reports to ACS, presentations in ASME, ACS, invited talks/seminars, and publications in high-quality academic journals.
One Phd student is supported by this grant and had plenty of hands-on learning experiences in nanomaterials processing techniques by working toward his dissertation. Till now, two journal papers have been submitted. One undergraduate student is recruited this semester to work on this project. The research achievements have been incorporated into the undergraduate class ME4330 (Mechanics of Nanomaterials), and graduate class ME5340 (Elasticity).
Copyright © 2014 American Chemical Society