58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND652648-ND6: Solvation Dynamics in Ionic Liquids
Steven A. Corcelli, University of Notre Dame
An important question remains for future studies: how
general is this anion-translation mechanism? However, as one deviates from the
structural motifs of the C153/[imidazolium][BF4] system, new
solvation dynamics mechanisms could emerge. This presents both challenges and
opportunities for the theoretical and experimental communities interested in
designing ILs with properties selectively tuned for specific applications.
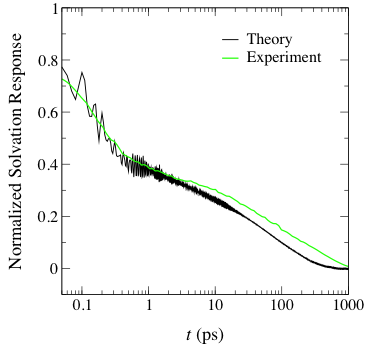
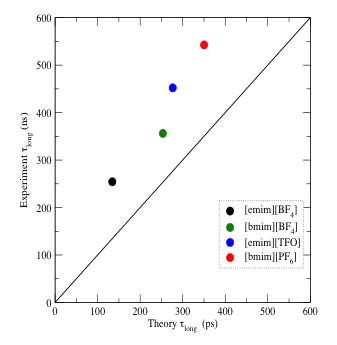
Figure 2. Comparison between experiment and theory of
the longest timescale for solvation dynamics in a series of imidazolium-based
ILs.
Copyright © 2014 American Chemical Society











