58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI151545-DNI1: Organocatalyzed Electroorganic Synthesis
Andrew J. Boydston, PhD, University of Washington
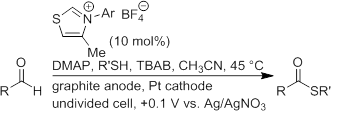
Building from our success with aldehyde-to-ester conversions via electro-organocatalysis (EOC), we decided to explore a broader scope of EOC capabilities starting with its application toward oxidative thioesterification. Few examples of NHC-catalyzed oxidative thioesterification of aldehydes are known in the literature. However, these approaches require exogenous oxidants such as phenazine, riboflavin, and azobenzene to achieve the desired redox transformation. Takemoto et al. discovered that the strength of the oxidant greatly influenced the thioesterification process due to unwanted oxidation of the thiol. Additionally, a related NHC-catalyzed method for the thioesterification of formylcyclopropanes provides acyclic thioesters via ring opening of the cyclopropane, concomitant formation of the acyl azolium species, and nucleophilic attack. An EOC approach would enhance chemoselectivity of the oxidation via Breslow intermediate formation and potentiostatic conditions would allow us to have precise control over the oxidizing potential.
Application of the EOC conditions developed for esterification only produced 26% yield of thioester in 23 h using p-tolualdehyde and 1-butanethiol. Upon analysis of the reaction mixture, disulfide formation was indicated as a major side reaction. It has been shown that thioesters can be formed from nucleophilic attack of disulfides by Breslow intermediates. However, control experiments revealed that under our reaction conditions, thioester is not formed via the disulfide mechanism. Additionally, investigations into the disulfide formation revealed that the catalyst was being deactivated in the presence of disulfide, consistent with sulfenylation of the NHC. Control experiments ruled out disulfide formation by electrochemical means. Following an extensive base screen, we found that the base strength and concentration strongly influenced the product distribution. Using 0.5 equivalents, relative to aldehyde, of 4-dimethylaminopyridine (DMAP) improved the yield to 73% in 27h (Table 1, entry 1). Electron-deficient aromatic aldehydes such as 3-methoxy-, 3-chloro-, and 4-fluorobenzaldehyde provided thioester in excellent yields under these reaction conditions (Table 1, entries 2 – 4). Less reactive aldehydes provided poor to moderate yields (Table 1, entries 5 – 8). These less reactive aldehydes require much longer reaction times allowing sufficient amounts of disulfide to form and “kill” the catalyst before achieving full conversion. These results led us to further investigate the source of the disulfide formation. When sodium thiolate salt was added to a solution containing 4-methyl-3-(2,4,6-trimethylphenyl)thiazolium precatalyst, disulfide formation occurred immediately. Additionally, no disulfide formation was observed when this experiment was repeated with thiol. Although the mechanism for this side reaction is currently unknown, the redox potentials for the precatalyst and thiolate anion suggest an electron transfer mechanism involving reduction of the precatalyst and oxidation of the thiolate is feasible.
Table 1. Oxidative thioesterification via EOC.a
Entry |
R |
R’ |
Ar |
Time (h)b |
Yield (%)c |
1 |
4-MeC6H4 |
Butyl |
Mes |
27 |
73 |
2 |
3-MeOC6H4 |
Butyl |
Mes |
29 |
96 |
3 |
3-ClC6H4 |
Butyl |
DiPP |
29 |
80 |
4 |
4-FlC6H4 |
Butyl |
Mes |
24 |
90 |
5 |
Cinnamyl |
Butyl |
Mes |
7 |
21 |
6 |
2-fur |
Butyl |
Mes |
22 |
30 |
7 |
Cyclohexyl |
Butyl |
Mes |
27 |
55 |
8 |
2,4-Me2C6H3 |
Butyl |
Mes |
31 |
66 |
9 |
3-MeOC6H4 |
2-fur |
Mes |
18 |
17 |
10 |
3-MeOC6H4 |
Cyclohexyl |
Mes |
38 |
55 |
11 |
3-MeOC6H4 |
Bn |
Mes |
31 |
62 |
aReactions conducted in dry CH3CN (15 mL) at RT in a 3-neck flask under N2 with a graphite anode, Pt basket cathode, 0.150 M RCHO, 0.305 M RSH, 0.075 M DMAP, 0.015 M thiazolium salt, 0.045 M TBAB, and constant cell potential of +0.1 V (vs Ag/AgNO3). Mes = 2,4,6-trimethylphenyl, DiPP = 2,6-diisopropylphenyl. bReactions were monitored by 1H NMR spectroscopy until thioester production had ceased. cDetermined by 1H NMR spectroscopy and GC-MS using 1,3,5-trimethoxybenzene as internal standard.
Alongside the aldehyde screen, we examined the thiol substrate scope. As shown previously in the aldehyde screen, 1-butanethiol performed well with the electron-deficient aldehydes. However, all other thiols furnished poor to moderate yields even when coupled with reactive aldehydes such as 3-methoxybenzaldehyde (Table 1, entries 9 – 11). These thiols produced large amounts of disulfide under our initial conditions, and we expect that solving the disulfide issue will help these thiols reach higher conversions.
Although the organocatalyzed anodic oxidation of aldehydes to thioesters requires further optimization, it has been successful for electron-deficient aromatic aldehyde substrates and shows promising preliminary results for less reactive aldehydes. This EOC approach avoids the use of pre-activated acylating reagents, exogenous oxidants, and transition metal reagents. However, in order to achieve higher conversions for less reactive substrates, disulfide formation must be suppressed. This side reaction operates via an unknown mechanism involving thiolate and NHC precatalyst. Consequently, we will investigate the mechanism by which disulfide is being formed and expand the scope of thioesterifcation via EOC.
Copyright © 2014 American Chemical Society