58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1050631-DNI10: Novel Nanocomposite Materials for Efficient Photocatalytic Reduction of CO2 to Fuels
Ying Li, University of Wisconsin (Milwaukee)
Objective
Photocatalytic reduction of CO2 with water by sunlight provides a new opportunity for simultaneous mitigation of greenhouse gases and production of energy-bearing compounds that can be further converted to substitutes for petroleum products. However, a low CO2 conversion rate and the lack of efficient and inexpensive catalysts have impeded the development of this technology. The objective of this research was and to design novel nanostructured composite materials to achieve high CO2-to-fuels conversion efficiencies and to understand the mechanism of CO2 photoreduction with water.
Results
For the previous reporting period (1/1/2011 ¨C 8/31/2012), we have developed three novel materials for CO2 photoreduction: (1) Cu-modified TiO2 nanoparticles with engineered surface defect sites; (2) Ag-deposited TiO2 nanoparticles assembled to form porous microspheres; (3) Ce-doped TiO2 dispersed on ordered mesoporous SBA-15 support.
For the current reporting period (9/1/2012 ¨C 8/31/2013), we have fabricated new nanocomposites and further advanced the study in CO2 photoreduction with water from the following two aspects: (1) Tailoring Cu valence and oxygen vacancy in Cu/TiO2 catalysts and understanding their effects in CO2 photoreduction, (2) Synthesizing porous microspheres of MgO patched TiO2 and studying the temperature dependent activity and stability for MgO/TiO2 catalysts
(1) Tailoring Cu valence and oxygen vacancy in Cu/TiO2 catalysts
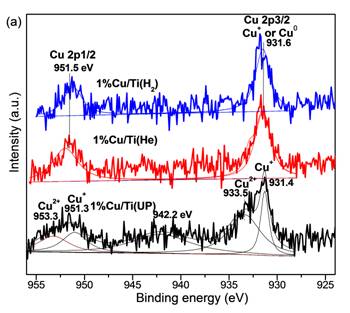
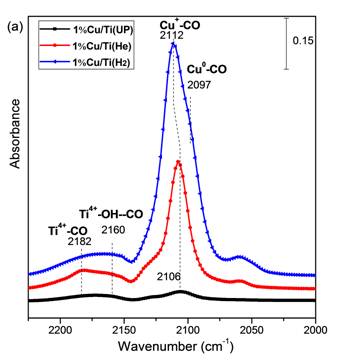
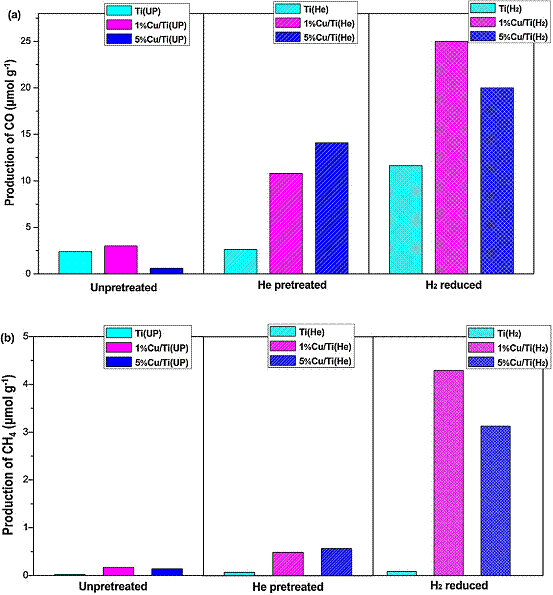
The incorporation of Cu species in TiO2 photocatalysts is beneficial to photocatalytic CO2 reduction to fuels, but the effect of Cu valence is poorly understood. In this work, Cu/TiO2 (P25) nanoparticle catalysts were prepared by a simple precipitation and calcination method. The as-prepared Cu/TiO2 sample was dominated by Cu2+ species. Thermal pretreatment of the as-prepared samples in He and H2 atmosphere resulted in the transition to a surface dominated by Cu+ and mixed Cu+/Cu0, respectively. The Cu valences were confirmed by in situ X-ray photoelectron spectroscopy (XPS) (Figure 1) and diffuse-reflectance infrared Fourier transform spectroscopy (DRIFTS) analyses (Figure 2). These thermal pretreatments in reducing atmospheres also induced the formation of defect sites such as oxygen vacancies and Ti3+, confirmed by UV-vis spectra and DRIFTS analyses. The various Cu/TiO2 catalysts were tested in CO2 photoreduction with water vapor under simulated solar irradiation, and their activities were in the order of as-prepared (unpretreated) < He-pretreated < H2-pretreated. Compared with unpretreated TiO2 (P25), the H2-pretreated Cu/TiO2 demonstrated a 10-fold and 189-fold enhancement in the production of CO and CH4, respectively (Figure 3). This significant enhancement was mainly attributed to the synergy of the following two factors: (1) the formation of surface defect sites promoting CO2 adsorption and subsequent charge transfer to the adsorbed CO2; (2) the existence of Cu+/Cu0 couples that facilitate electron and hole trapping at different sites.
(2) Porous microspheres MgO patched TiO2 for enhanced activity and stability
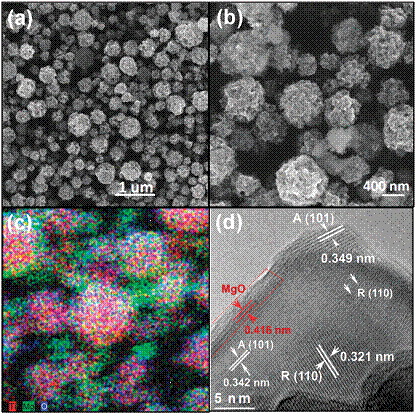
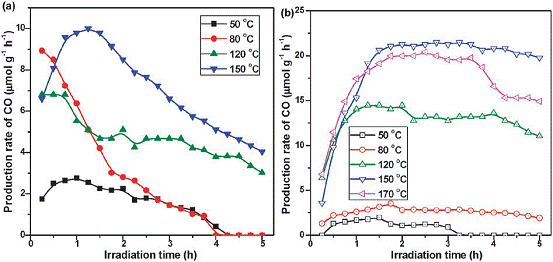
A novel MgO-patched TiO2 microsphere photocatalyst (Figure 4) was fabricated through a simple ultrasonic spray pyrolysis method using TiO2 (P25) nanoparticles and Mg(NO3)2 as the precursors. Conventional photocatalysis experiments are conducted at room temperature; in this work, we have investigated the effect of reaction temperature in the range of 50 to 170 oC. As shown in Figure 5, key results from this work are: (1) the activity of CO2 photoreduction to CO increased as the temperature increased for both TiO2 and MgO/TiO2, and reached optimum at 150 oC; (2) the incorporation of MgO to TiO2 enhanced both production yield and the stability of the catalyst. The possible reason for the superb activity at 150 oC for MgO/TiO2 is easier desorption of reaction intermediates and the enhanced CO2 adsorption by MgO. A higher temperature favors intermediates/products desorption but limits CO2 adsorption, as confirmed by in situ DRIFTS analysis data. The weakened CO2 adsorption ability, however, is compensated by adding MgO to the TiO2 surface. The finding in this work is of great importance as it provides a new reaction scheme that may be suitable for power plant flue gas CO2 capture and conversion where the temperature is in the range of 130 to 150 oC.
Impact
This two-year research supported by ACS-PRF has resulted in a total of five journal articles published in Journal of Physical Chemistry C, International Journal of Hydrogen Energy, and Catalysis Science & Technology, Applied Catalysis B: Environment, and Chemical Communications, respectively. The results were also disseminated in several national conferences. The findings of this project have contributed to the field of solar fuel production by providing significant insights in materials design and reaction mechanism. The ACS-PRF project has trained one postdoc and two graduate students. It also provided research experience for one undergraduate student who also co-authored some of the journal publications. The ACS-PRF grant has served as important seed funding for the PI to secure other funding to conduct research in the field of renewable energy, including a recent NSF CAREER Award in 2013.
Figure 1. In situ XPS spectra for Cu 2p of 1%Cu/TiO2 samples
Figure 2. In situ DRIFTS spectra of CO adsorption on unpretreated and pretreated 1%Cu/TiO2 sample;
Figure 3. The production of (a) CO and (b) CH4 on the unpretreated and pretreated TiO2 and Cu/TiO2 catalysts under photoirradiation for 6.5 hr.
Figure 4. SEM images (a, b), X-ray elemental mapping image (c), and HRTEM image (d) for MgO/TiO2 catalysts.
Figure 5. Rate of CO production from CO2 photoreduction with H2O vapor on (a) TiO2 and (b) MgO/TiO2 at different reaction temperatures
Copyright © 2014 American Chemical Society