58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI452165-UNI4: Proton-Coupled Electron Transfer in Ground-State Charge Transfer Reactions: Bi-molecular PCET and Intervalent Charge Transfer
Eliz Young, PhD, Amherst College
Management of charge transfer is a fundamental problem that the work of this grant aims to address. Charge balance must be maintained as certain chemical reactions progress, meaning that movement of an electron to and away from a reaction site may be coupled to shorter proton movement. This mechanism is known as proton-coupled electron transfer (PCET). Coupling of electron and proton motion has far-reaching implications for the numerous chemical systems that undergo multi-proton, multi-electron transformations. PCET has been revealed to be a critical component in the chemical activation of substrate bonds at catalytically active sites as well as in amino acid radical generation and transport through protein matrices. Specifically, this work seeks to advance our understanding of charge transfer and proton management in fundamental, model systems.
Two simplified PCET mechanisms describing the direction of the proton (PT) and electron (ET) transfer with respect to each other are unidirectional PCET and bidirectional PCET (Scheme 1). Unidirectional PCET occurs when ET and PT occur along the same coordinate. Bidirectional PCET describes systems in which the proton and electron travel along different coordinates.
Scheme 1: PCET mechanicsms. De and Ae are the electron donor and acceptor and Dp and Apare the proton donor and acceptor, respectively.
Model System: Work in this proposal focuses on the model compound, Fcam1 (Scheme 2), which has been designed for the mechanistic study of bidirectional PCET. Specifically, the structure of Fcam1accentuates the effect of proton motion, marked by the protonation state of the amidinium functionality, on movement of electrons in the small-molecule ferrocene unit. The amidinium proton handle and the ferrocene redox center are very highly coupled supporting the intended goals for this model system.
Scheme 2: Ferrocenyl amidinium.
Results from Year 1
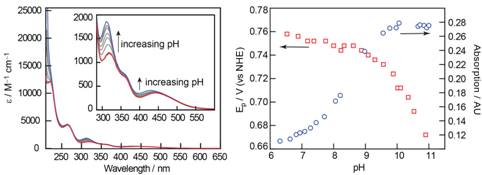
UV-visible absorption spectroscopy (Figure 1, left) and electrochemical experiments (data not shown) performed on Fcam1 reveal marked variation with pH. The spectral and electrochemical behaviors are closely correlated. Oxidation potentials plotted versus pH (Figure 1, right) track the spectral shifts observed in UV-visible spectra (Figure 1, right) thereby confirming that oxidation of the Fcam1 is gated by deprotonation of the amidinium functionality. A pKa value of 8.8 for Fcam1is determined from the plots in Figure 1.
Figure 1: Left) pH-dependent UV-visible absorption spectra of Fcam1as a function of increasing pH (red, pH 7; blue, pH 11). Shifts in the spectra are clearly seen as increasing pH causes deprotonation of the pendant amidinium. Right) The spectral shift at 450 nm (blue, circle) and the oxidation potentials (red, square) are plotted versus pH.
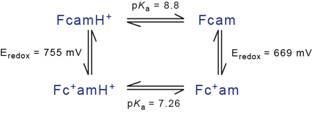
The thermodynamic properties of Fcam1 are summarized using a square scheme (Figure 2), for which complete labelling of acidity constants and oxidation potentials is presented. The pKa value for the protonated form of Fcam1 is experimentally determined, as are the oxidation potentials of both protonated and deprotonated Fcam1. With three of the four values experimentally determined, the square is completed by calculating the pKa value for the ferrocenium-amidinium species, Fcam1+. The value obtained from these calculations is pKa(Fcam1+) = 7.26. This square scheme forms the basis for designing a D-A system for bi-directional PCET studies involving Fcam1.
Figure 2: Thermodynamic square scheme for Fcam1that fully characterizes the protonation states and redox potentials in aqueous conditions.
We attempted to design a PCET experiment with a ground-state, diffusion-controlled PCET mechanism. This type of thermally activated PCET experiment was intended to serve as starting point for incorporating Fcam1into more complex, covalently bound systems.
Significant effort was invested into finding a suitable chemical oxidant for the Fcam1 moiety. For this experiment, a suitable oxidant, and its reduced radical anion, must be stable in basic aqueous conditions both above and below the pKa of Fcam1, specifically in the range of pH 7 – 10. To our disappointment, this stringent stability requirement has precluded demonstration of ground-state diffusion controlled PCET with Fcam1. While commonly used at low pHs (below pH 7), appropriate chemical oxidants for this process suffer from instability at high pH conditions. Therefore, to demonstrate pH-dependent PCET, it will necessary to move away from a diffusion-controlled experiment and to incorporate Fcam1into a covalently bound D-A system, in which ET is initiated from the excited state of a photo-oxidant. We are currently considering molecular design of such a system.
Impact on Faculty and Students
Four undergraduate students have been supported. Ewuradjoa Gadzanku ’13 stayed after her senior thesis to complete several unfinished studies. Claire Drolen ’15 has been supported for work during her sophomore academic year (AY 2012-2013). Stephen Hetterich ’15 and Sam Hendel ’15 were supported during the summer of 2013. Claire, Stephen and Sam will continue their work this academic year (AY 2013-2014).
These undergraduate researchers have been exposed to fundamental aspects of charge transfer mechanisms and various experimental techniques including UV-visible absorption and fluorescence spectroscopy, electrochemistry, pKatitrations. Stephen will travel to M.I.T. in September 2013 to carry out time-resolved kinetics measurements necessary for this project.
Ewuradjoa and the PI attended the ACS green chemistry conference in June 2013. Stephen, Sam and the PI will attend the local (Northeast Regional) ACS meeting in October 2013 where both Sam and Stephen have submitted abstracts to give oral presentations in the undergraduate session.
Integrating Research and Teaching
Sam’s project involved developing a physical chemistry teaching laboratory based on PCET, catalysis and electrochemistry, which are key components of the research under this grant. The laboratory will be implemented the next time the PI teaches this course at Amherst. This fall, the laboratory will be implemented at University of Texas El Paso under the direction of Prof. Dino Villagran. After running the laboratory serveral times, the laboratory protocol will be submitted for publication to the ACS Journal of Chemical Education.
The teaching laboratory will also serve as a tutorial for any student wishing to join the PI’s lab in the future as a formative aspect of their training in electrochemistry, a technique vital to research under this grant. Thus, Sam’s work will impact students in the teaching and research enviroment at Amherst.
Copyright © 2014 American Chemical Society