58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR552145-UR5: Synthesis of Ruthenium Dioxide Nanoparticles and Clusters with Tailored Size-and Surface-Dependent Properties for Enhanced Catalytic Applications
Vladimir Kitaev, Wilfrid Laurier University
Technical report of the progress (July 2012- May 2013)
At the initial stages of the project, nanostructures (small nanoparticles and clusters composed of few tens of the atoms) based on iridium dioxide and ruthenium dioxide have been prepared using several stabilizing ligands. The main tested ligands were captopril ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl] pyrrolidine-2-carboxylic acid) and guanidine. Captopril was chosen as thiol and carboxylate-bearing molecules (negatively charged species) Arginine was selected as a highly basic amine (amino groups and potentially serving as positively charged species).
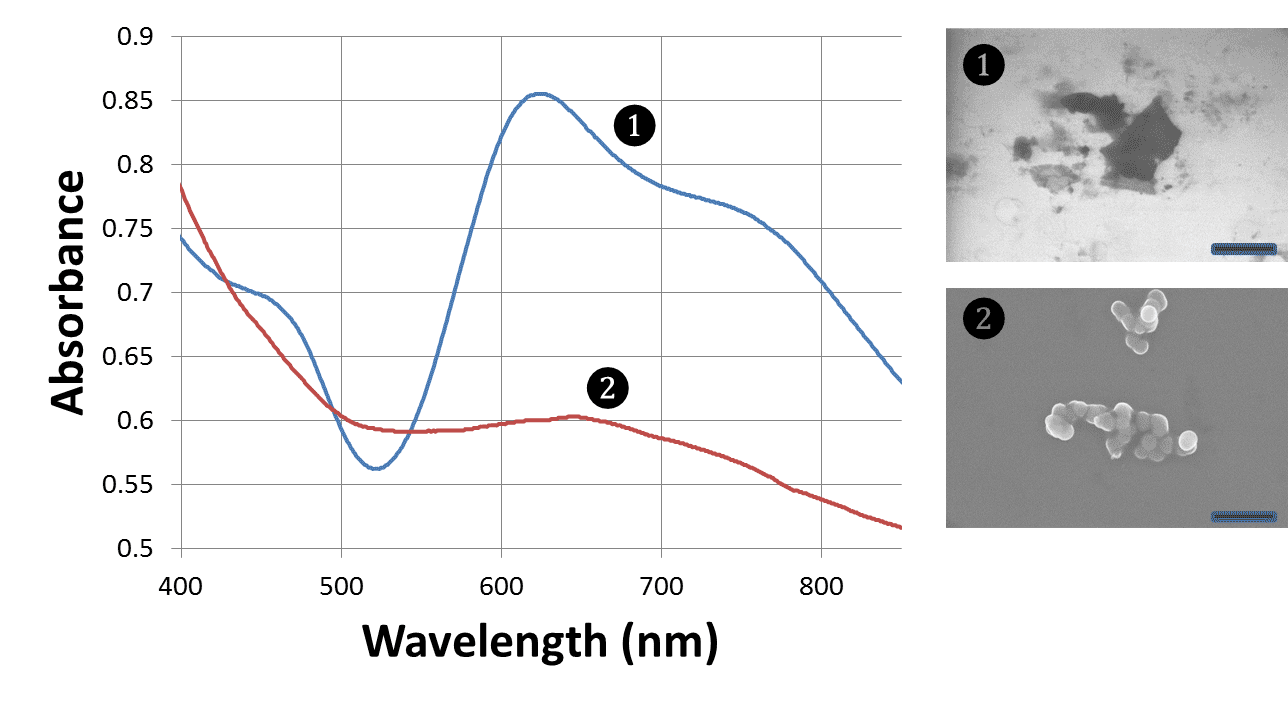
Figure 1. UV-vis spectra and EM images of Ir(IV) oxide nanostructures. Both samples are stabilized with captopril and guanidine. Sample 1 (TEM) is produced by light exposure and features sheet-like morphology; while sample 2 (SEM) is produced by heat treatment and is more isotropic. Scale bars are 1.5 µm.
We have been able to prepare nanostructures based on iridium oxide. Two main different morphologies produced were sheet-like and spherical aggregates (Figure 1). Both of these forms display strong optical absorption that is characteristic of electron-rich species. Photoelectrochemical activity of these nanostructures as an anode for water splitting (using white, violet and blue lights, base electrolyte, Pt cathode, with the potential applied ranging from 0.3 to 0.7 V) was screened and has been found in line with the values previously reported in literature.
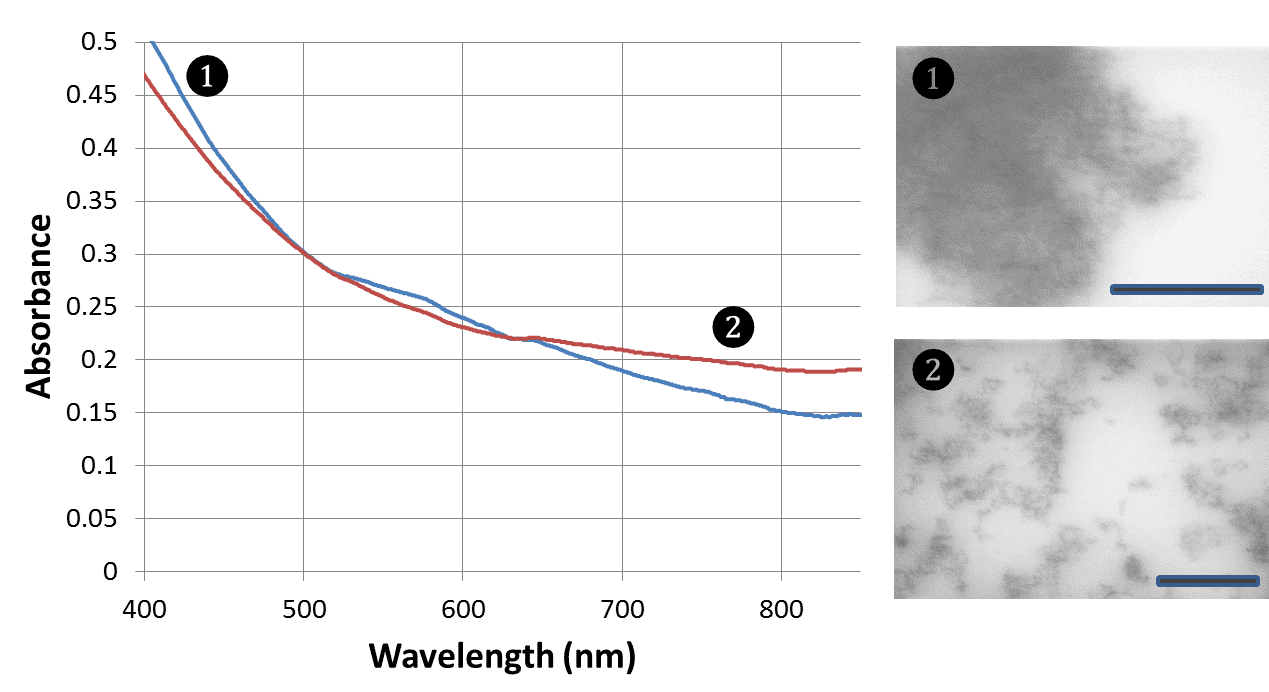
Figure 2. UV-vis spectra and TEM images of RuO2 nanostructures (nanoclusters). Both samples are stabilized with captopril. Scale bars are 500 nm.
Similarly to iridium dioxide, optically active ruthenium oxide have been successfully prepared. For these materials, a synthetic emphasis was placed on preparation of small (few tens of atoms) cluster-like nanostructures. We have been able to achieve this goal (Figure 2). Photoelectrochemical activity of these ruthenium dioxide nanostructures has been tested and found comparable or superior to the previously reported data.
Encouraged by the promising photoelectrochemical results, approaches to practical implementation of anode materials for water splitting have been thought. There it was immediately realized that the cost-effectiveness of these materials is of primary importance (e.g. to compete with silicon cells).
Other non-costly electron-rich oxide materials have been approached, in particularly manganese (IV) oxide. It has been also decided to prepare and test hematite-based materials, given our previous experience with their synthesis.
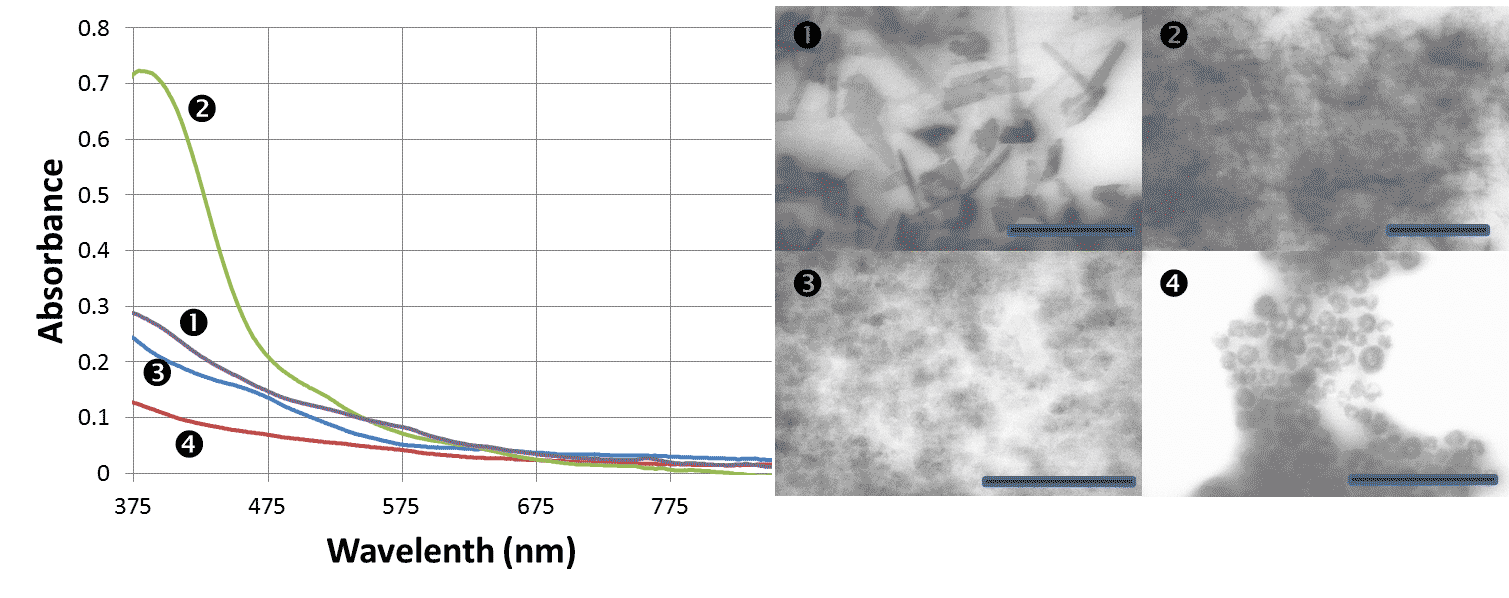
Figure 3. UV-vis spectra and TEM images of various morphologies of MnO2 nanoparticles.
1- sheet/rolled like morphology, 2- crumpled sheet like morphology,
3- sub-nanoparticle/cluster like morphology, 4- shell like morphology.
Scale bars are 500 nm for 1&2 and 400 nm for 3&4.
Preparation of sheet-like manganites and manganese oxide has been successfully developed (Figure 3). These materials show very promising electrochemical properties.
Figure 4. UV-vis spectra and EM images of iron oxide nanoparticles. Spectra corresponds to the images. 1 and 2 TEM images of hematite (Fe2O3 ) nanoparticles. 3 SEM image of hematite, scale bar is 500 nm. 4 TEM image of underdeveloped hematite, containing akaganéite (β-FeO(OH)). Scale bars are 500 nm for 1, 3 and 4, and 400 nm for 2.
Similarly hematite and related oxides has been prepared in different morphologies (Figure 4) with the activity similar or slightly superior to the similar previously reported materials.
In the current stage of the project, different doping and mixed oxide systems are actively investigated to capitalize on our synthetic capabilities and understanding of colloidal nanoscale materials.
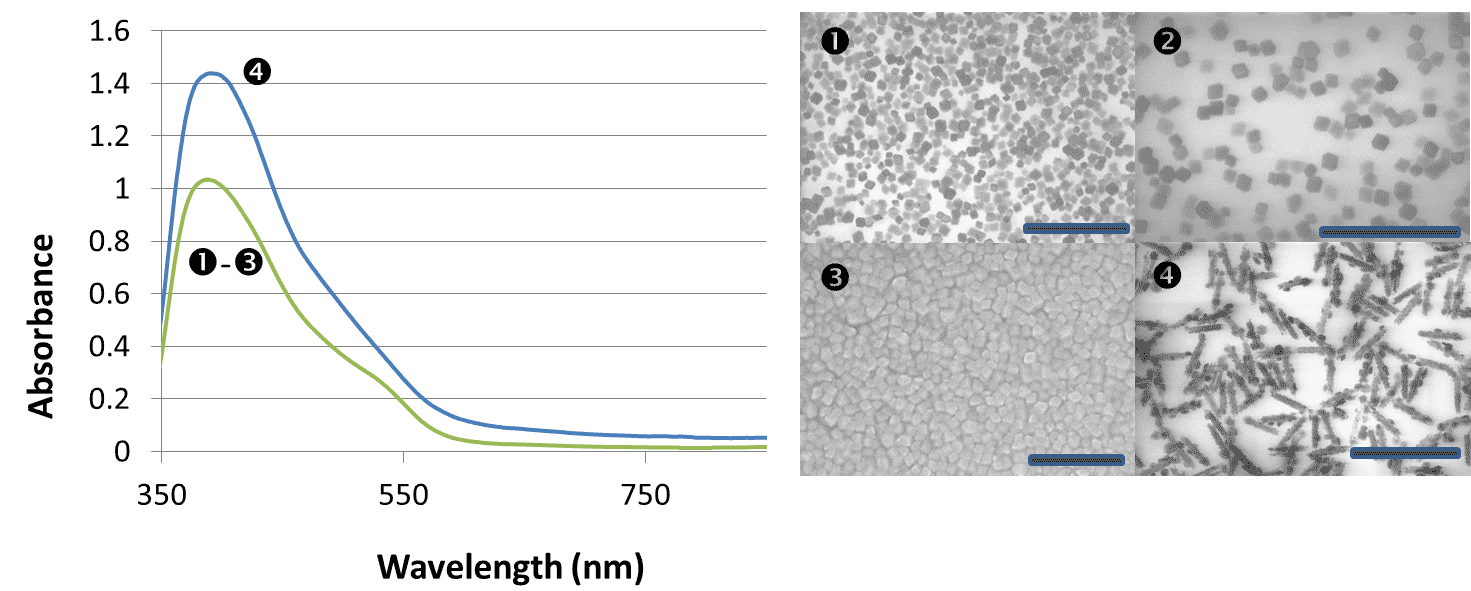
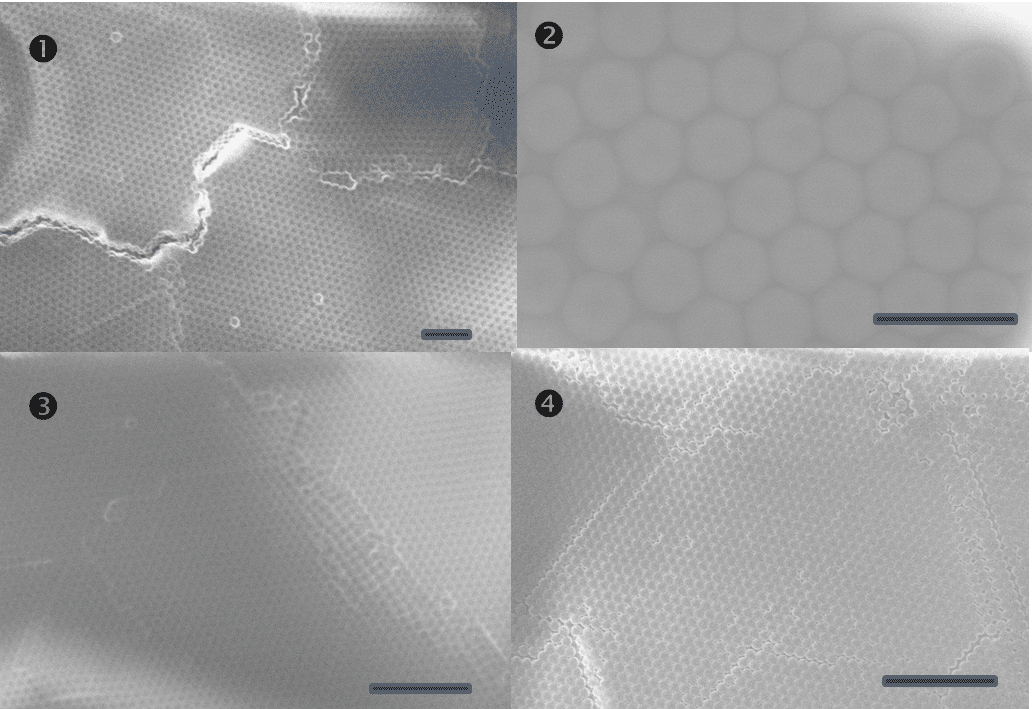
Figure 5. SEM images of monodisperse positively-charged polystyrene latex. Scale bars are 2.5 µm in 1, 3 and 4, and 500 nm in 2.
An appreciable time (equivalent of ~6 months of a full-time undergraduate student work) has been also devoted to the preparation of positively charged latexes (Figure 5) to approach controllable porosity of the anode materials if it may become necessary in the project.
Overall, the project progresses well, especially given the changes in the main direction and appreciably novel and challenging goals.
Copyright © 2014 American Chemical Society