58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI1051028-DNI10: Enhanced Chromophore Stability Using Fluorescence Quenchers for Organic Photovoltaic Devices
Samuel W. Thomas, PhD, Tufts University
Although remarkable gains have been made in the efficiency and stability of organic semiconducting devices, the current infeasibility of these materials require further improvements to their performance and cost. Controlling these materials in the solid-state remains a persistent challenge.1 While the interactions between alkyl side-chains is important to the solid-state organization of polymers, using them to program behaviors in the solid-state is new. Toward the goal of understanding how chemical structure of side-chains can influence the properties of these materials, we report here progress on two projects: 1) the interactions between side-chains and main-chains to influence the bandgaps of conjugated materials, and 2) photocleavable side-chains to yield conjugated polymers that behave as negative photoresists.
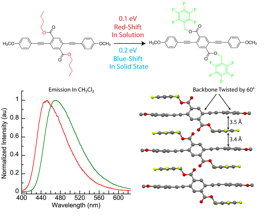
In work that is currently submitted for publication, we have discovered several examples of conjugated oligomers and polymers in which the side chains that are formally not in conjugation with the conjugated backbone play a critical role in the band gap of these materials. The most striking of these examples is the difference in solid-state optical properties of the oligomers shown in Figure 1: compared to the conjugated oligomer with alkyl esters, the absorbance and emission spectra of the pentafluorobenzyl ester-substituted oligomer shows a 0.1 eV red shift in solution; this shift is due to inductive strengthening of the donor-acceptor character of this molecule by the inductively electron-withdrawing fluorine atoms. In the solid state this trend reverses, and the pentafluorobenzyl oligomer is 0.2 eV blue-shifted from the alkyl-ester substituted oligomer. X-ray crystallography reveals this hypsochromic shift is due to arene-arene cofacial interactions between the perfluorinated side chain and the main chain anisyl rings twisting the main chain out of conjugation. Poly(phenylene-ethynylene) derivatives of this twisted chromophore resist planarization and/or chromophore aggregation upon spin-casting.
Figure 1. Due to cofacial interactions in the solid state, molecules with heavily fluorinated benzylic esters show a hypsochromic shift in the solid-state while using the same conjugated backbone.
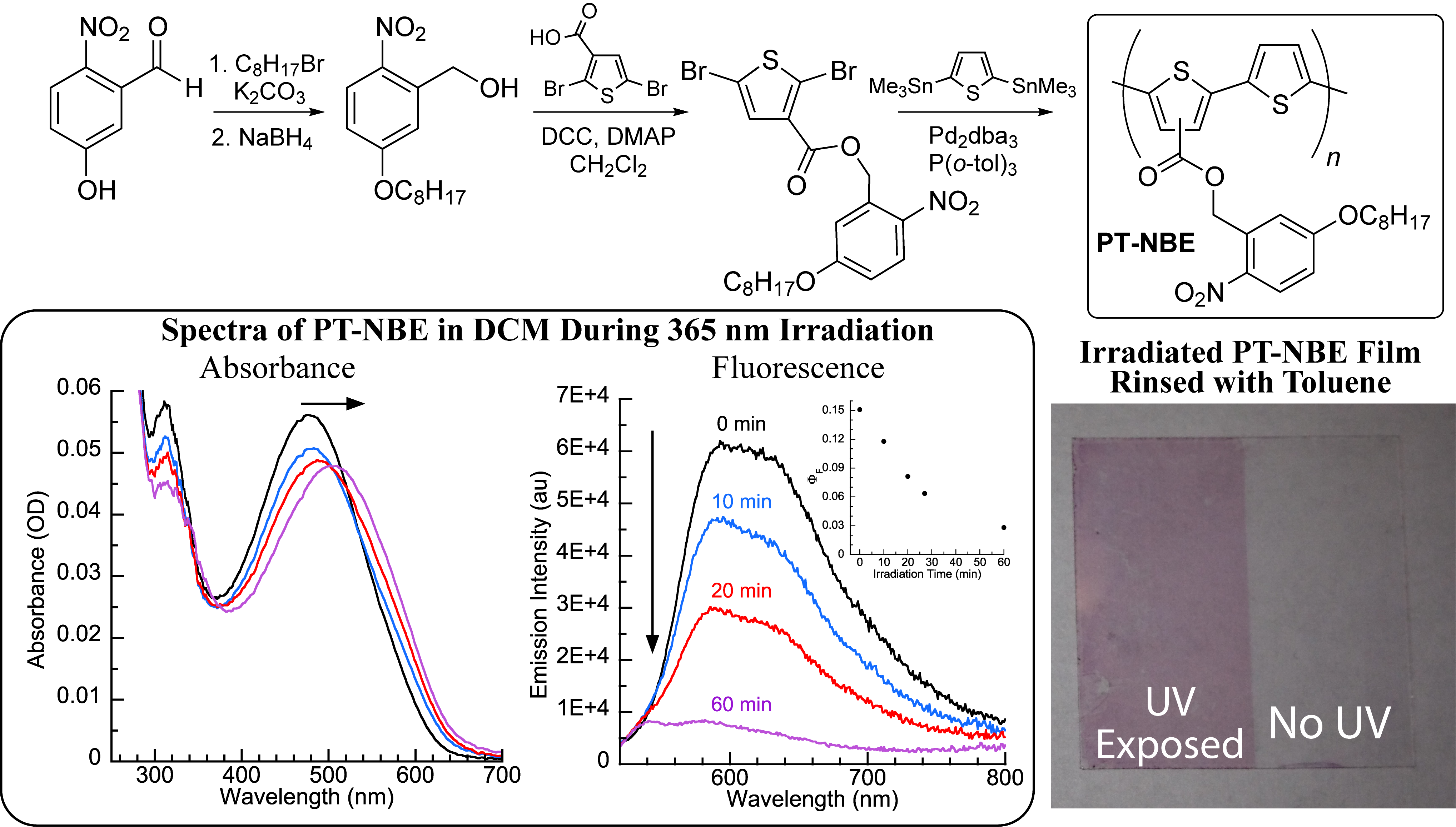
In work in last year's PRF narrative report,2 conjugated polymer PT-NBE aggregated in solution upon UV-induced photolysis of solubilizing side-chains (Figure 2). Upon irradiation of a spun cast film of the PT-NBE, only 10-15% the polymer film was toluene-soluble.
Figure 2. Results from 2011-2012: Top: Synthesis of PT-NBE. Bottom Left: Irradiation of a dilute CH2Cl2 solution of PT-NBE at 365 nm yields red-shifted absorbance and quenched fluorescence. Bottom Right: Irradiation of a spun-cast thin film of PT-NBE leads to a toluene-insoluble polythiophene film.
This year, we established two important structure-property relationships of our polymers:
1) Nitrobenzyl ethers are about 10x more efficient in photocleavable than nitrobenzyl esters. Prompted by a study in the primary literature concerning how the efficiency of nitrobenzyl photolabile groups correlates with radical stabilization energy (RSE) of the corresponding nitrobenzyl radical,3 we measured the quantum yield of photolysis of the nitrobenzyl groups in the two molecules (a nitrobenzyl ether and a nitrobenzyl ester) in Figure 3 by 1H NMR. Our results indicate that in conjunction with the reported trend, the ether photolysis had a ~10x larger quantum yield than the ester.
Figure 3. Structures of conjugated oligomers bearing photocleavable nitrobenzyl esters or ethers, with ethers showing significantly greater quantum yields
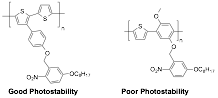
2) Photostability correlates with chemical structure. An important consideration in these studies is the photostability of the material. We studied the photochemical reactions of two new polymers, shown in Figure 4, in both solution and the solid-state. For the sake of synthetic accessibility, we prepared polymers that had nitrobenzyl ether groups as more efficient aryl ethers. The poly(thiophene-phenylene) with methoxy groups that are not photoreactive showed rapid photodegradation, while the polythiophene without such groups showed much greater photostability, consistent with empirical guidelines reported by Krebs and coworkers.4 These polymers also behaved as negative photoresists.
Figure 4. Photolabile nitrobenzyl ether-containing conjugated polymers photoresists and their relative photostability in the solid-state.
1. Henson, Z. B.; Mullen, K.; Bazan, G. C. Nat. Chem. 2012, 4, 699-704.
2. Smith, Z. C.; Pawle, R. H.; Thomas, S. W. ACS Macro Lett. 2012, 1, 825-829.
3. Solomek, T.; Mercier, S.; Bally, T.; Bochet, C. G. Photoch Photobio Sci 2012, 11, 548-555.
Copyright © 2014 American Chemical Society