58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR450664-UR4: Synthesis of Diarylnorbornadiene Derivatives and their Potential Use in Solar to Thermal Energy Conversion
Felix E. Goodson, PhD, West Chester University of Pennsylvania
Karyn Usher, PhD, Metropolitan State University
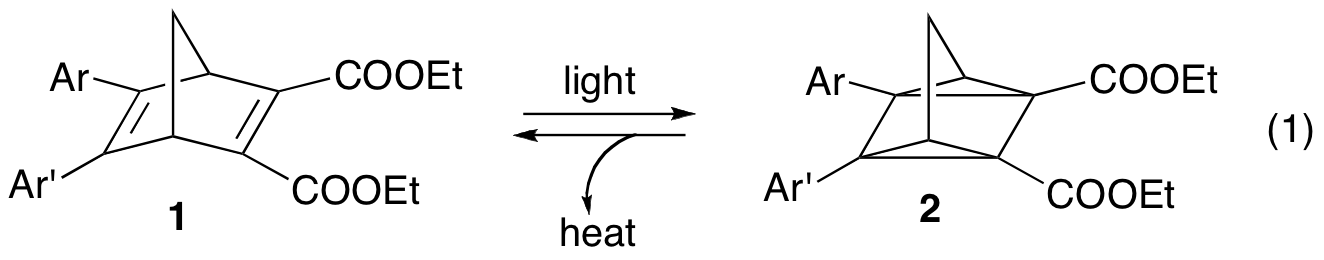
Our research has focused on the synthesis of a series of diarylnorbornadiene compounds (1). These compounds are of interest because the norbornadiene can undergo a [2+2] cycloaddition to form the highly strained quadricyclane (2) (eq. 1). Since this strain energy could later be released in the form of heat, these compounds have been envisioned for applications in the area of solar to thermal energy conversion.1 The aromatic rings and ester groups provide for absorption at longer wavelengths and a means of promoting the reversion with acid catalysis.2
The traditional means of synthesizing these compounds (developed by Hirao in 1984) relied on a multistep route.2 However, we have developed a two-step procedure from readily available starting materials (Scheme 1). In this manner we have synthesized and characterized a library of sixteen diarylnorbornadiene derivatives in which groups on one of the aromatic rings have been independently varied.
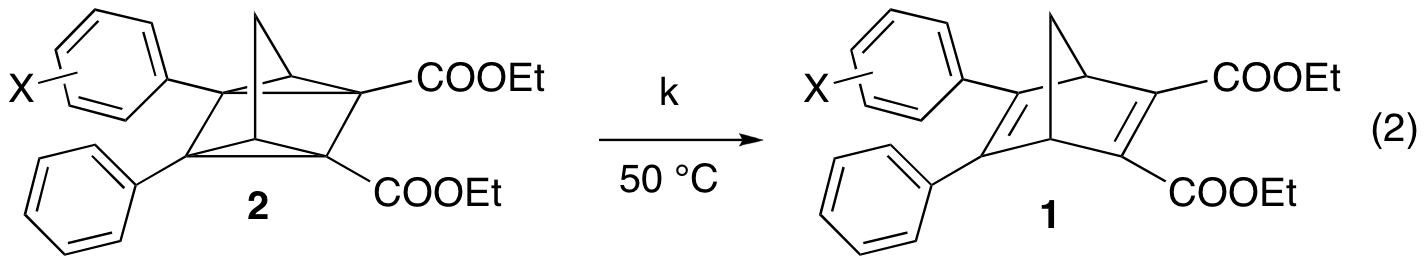
With this library of compounds in hand, we next performed experiments to study how substituents on one or more of the benzene rings affect the thermal stability of the quadricyclanes. By monitoring the kinetics of the reversion (eq. 2) via UV-Visible spectroscopy, we determined that electron-withdrawing groups in the meta position slowed down the reversion, and thus had a stabilizing effect on the quadricyclanes. Electron-donating groups, as well as groups that provide conjugation in the para position, exhibited a corresponding destabilizing effect. A Hammett-style analysis utilizing sigma constants from hyperfine splitting of substituted benzylic radicals4 resulted in a reasonable linear fit, suggesting that the quadricyclane to norbornadiene reversion follows a free-radical pathway. While this has long been considered to be the case,5 we now have some experimental data in support of this assertion.
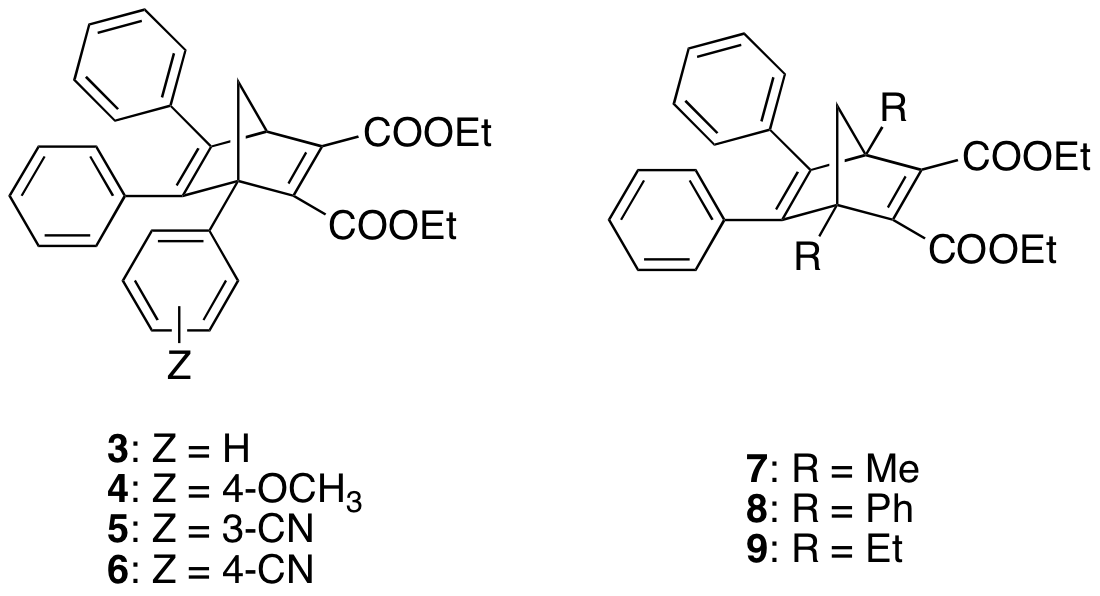
Unfortunately, none of the derivatives in our first library formed a stable quadricyclane, unlike some of the compounds originally presented by Hirao.2 The key difference is the presence of methyl groups at the bridgehead positions (which were a requirement in the original multistep synthesis). Could these methyl groups be having such a profound effect on the stability of the quadricyclanes? To investigate this question, we next synthesized compounds 3-6, in which we varied substituents on an aryl group at the bridgehead position, as well as compounds 7-9, with different groups at both bridgehead positions. We then studied the kinetics of the reversion of the corresponding quadricyclanes.
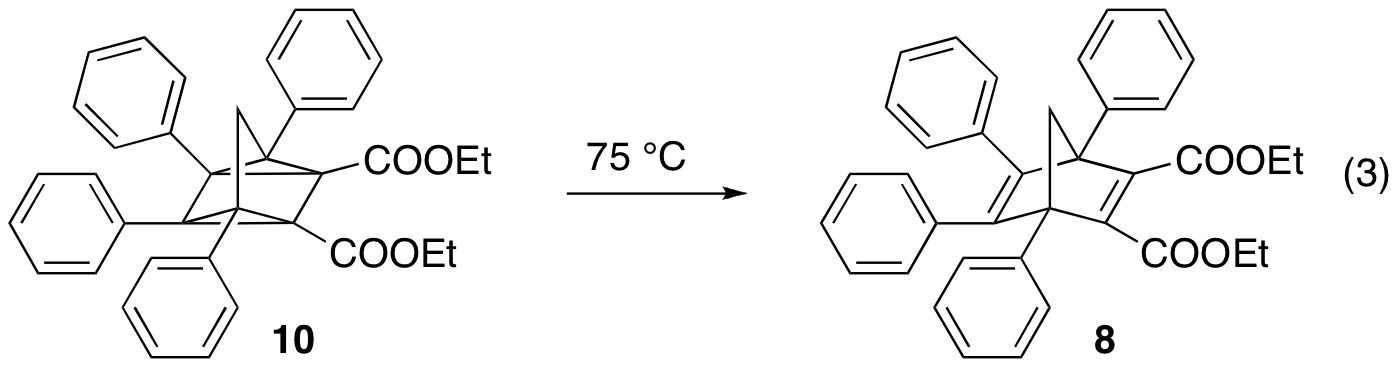
Somewhat surprisingly, substituents on the bridgehead aryl of compounds 3-6 had very little effect on quadricyclane stability. On the other hand, the identity of the substituents in 7-9 had a profound effect, with ethyl groups stabilizing the quadricyclane more than phenyl, which was in turn a better stabilizer than methyl. This trend was a bit of a surprise. If sterics were the determining factor, one would expect the phenyl substituent to provide the most stable quadricyclane. If electronic variation at the bridgehead position was more important, then the methyl and ethyl derivatives should behave similarly. Thus, at this point we believe quadricyclane stability to be governed by a mixture of sterics and electronics. Present work is geared towards the synthesis of derivatives with bulkier groups in order to explore this idea further. One more ominous aspect that came to light in these studies is that the reversion of quadricyclane 10 (eq. 3) was not clean, resulting in several products in addition to 8. This suggests another parameter that needs to be screened in our search for the optimal norbornadiene to be used for solar energy storage.
To date, summer research opportunities for ten undergraduate students have been fully or partially supported by this grant. Of the six that have already graduated, two are currently pursuing Ph.D. degrees in chemistry and two others are enrolled in graduate programs in other disciplines. Of the four remaining students, three hope to obtain a graduate degree in chemistry, while the fourth is planning on applying to medical school. Five of the students have had the opportunity to present their work on this project at recent American Chemical Society national meetings. All ten of these students have learned skills in organic synthesis, liquid chromatography, and the analysis of kinetics data.
In terms of the impact of this research on the careers of the principal investigators, the technology of chromatography has come a long way since the days of drip columns, which were prevalent in the era when one of us (Dr. Goodson) was in graduate school. The work in this project would not have been possible without the collaboration with Dr. Usher, whose expertise has enabled all of us involved to learn about the advances in liquid chromatography, as well as the skills and instrumentation required to perform the challenging separations. Involvement in this research has had an impact on Dr. Usher's career by introducing her to organic techniques and methodologies that she had not previously experienced. The interaction and collaboration with Dr. Goodson and the undergraduate researchers has been a very positive experience.
References.
1. Dubonosov, A. D.; Bren, V. A.; Chernoivanov, V. A., Russian Chem. Rev. 2002, 71, 917-927, and references cited therin.
2. Hirao, K.; Ando, A.; Hamada, T.; Yonemitsu, O., J. Chem. Soc., Chem. Commun. 1984, 300-302.
3. Shaulis, K. M.; Hoskin, B. L.; Townsend, J. R.; Goodson, F. E.; Incarvito, C. D.; Rheingold, A. L., J. Org. Chem. 2002, 67, 5860-5863.
4. Dust, J. M.; Arnold, D. R., J. Am. Chem. Soc. 1983, 105, 1221-1227.
5. Kabakoff, D. S.; BŸnzli, J.-C. G.; Oth, J. F. M.; Hammond, W. B.; Berson, J. A., J. Am. Chem. Soc. 1975, 97, 1510-1512.
Copyright © 2014 American Chemical Society