58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR351085-UR3: Fundamental Chemistry of the Re(CO)3(H2O)3+ Synthon
Richard Herrick, PhD, College of the Holy Cross
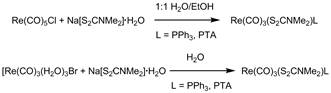
Low-valent technetium and rhenium dithiocarbamate compounds M = 99mTc, Re; S2CNR2 = dithiocarbamate, have been closely studied as possible diagnostic/therapeutic agents in medicine.1 We previously reported the syntheses and characteristics of the rhenium congeners of this family of the form, Re(CO)3(S2CNMe2)L, L = neutral ligand.2 These compounds were prepared in methanol. Competition studies showed that the PPh3 and PTA (PTA = 1,3,5-triaza-7-phosphaadamantane) derivatives, were especially stable in the presence of biological nucleophiles. We decided to extend this study and look at making the same compounds under aqueous conditions using a microwave reactor. Aqueous conditions work well with microwave reactors due to the superior ability of water to absorb microwave radiation. To our knowledge, a microwave reactor has not been utilized in d6 rhenium chemistry, but the common resistance of its compounds to hydrolysis and oxidation suggest that these reactions would be a good test of this technique. This procedure works very well, and each product was obtained in high yield with little purification required (Scheme 1).
Scheme 1. Syntheses of Re(CO)3(S2CNMe2)L from different rhenium reagents.
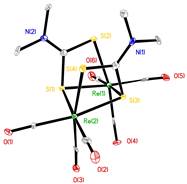
The reaction without neutral ligand present resulted in formation of a pure, colorless compound that readily gave crystals. Structural elucidation revealed the dimeric compound [Re(CO)3(S2CNMe2)]2 1 as seen in Figure 1. The structure is a distorted conlateral bioctahedron where one sulfur atom from each dithiocarbamate bridges the two metal centers while the other sulfur atom from each dithiocarbamate binds a single metal center. The dithiocarbamates each lie above the Re2S2 structure forming a cisoid geometry. The carbonyls adopt the usual facial geometry creating a C2 symmetric molecule. The molecule is chiral with stereochemistry imposed by the positioning of the ligands and the metal atoms as neither precursor is chiral.
Figure 1. Molecular structure of 1. Hydrogen atoms have been omitted.
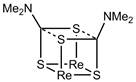
While the transoid geometry, with the bidentate ligands on opposite sides of the M2X2 quadrilateral, is more common than the cisoid geometry in these structures, the analogous technetium compound, [Tc(CO)3(S2CNEt2)]2, also adopts a cisoid geometry and is isostructrual to the structure reported here.3 The choice of cisoid geometry by two similar dimers suggests that the ligand plays a role in choice of geometry. Closer examination provide a possible explanation. An alternative way of describing the central unit of the molecule is as an open cubane with the central Re2S2 structure joined by the other two sulfur atoms and the two central carbon atoms. As shown in Figure 2, the structure lacks two bonds that would join the central carbon atoms to the opposing non-bridging sulfur atoms. However, this pair of non-bridging sulfur atoms and central carbons atom lie in close proximity; C(7)-S(2) = 3.255 Å and S(4)-C(1) = 3.214 Å. These values are within the sum of van der Waal radii for these atoms. The dithiocarbamate ligand when bound as a bidentate ligand has a partial negative charge localized on each sulfur, and the central carbon atom has a partial positive charge localized on it. Close interaction of opposite positive charges provides stabilization energy.
Figure 2. Idealized representation of the open cubane portion of 1. Dotted lines indicate non-bonding C-S interactions.
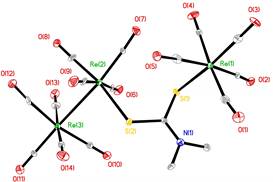
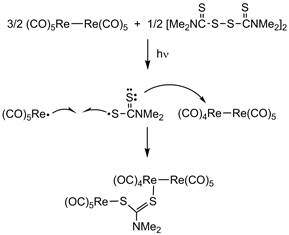
When the aqueous attempt to make Re(CO)4(S2CNMe2) yielded a different product, we turned our attention to a non-aqueous route. Its synthesis by photochemical treatment of Re2(CO)10 and tetramethylthiuram disulfide [Me2NC(S)S]2 was reported briefly by Brown and Lee.4 We repeated the synthesis using pentane as the solvent. Surprisingly, Re(CO)4(S2CNMe2) was not observed. The only organometallic product that was obtained was a light orange compound that was not Re(CO)4(S2CNMe2). Crystals of this compound were structurally elucidated as (CO)5ReSC(NMe2)SRe2(CO)9 2. The molecular structure is shown in Figure 3.
Figure 3. Molecular structure of 2. Hydrogen atoms have been omitted.
This complex captures the two primary photoproducts generated by M2(CO)10, M = Mn, Re, in a single compound. Previous research in the 1980s uncovered the details of each photochemical process photolysis generates both a 17-electron, metal-centered radical, (CO)5M×, and it also generates the electron deficient M2(CO)9 species. What is surprising here is that the product must have been produced from both intermediates. Apparently the tetramethylthiuram disulfide is converted to the dithiocarbamate radical in the reaction solution. It reacts both with Re(CO)5 and with Re2(CO)9 to create the observed product (Scheme 2).
Scheme 2. Proposed mechanism for formation of 2.
In summary, we find that that microwave reactions of aqueous solutions are an underutilized option for preparing d6 rhenium compounds and are pursuing further research in this area. The reactions are environmentally friendly and work as well or better than traditional methods. A new dimer, 1, was prepared while attempting to make Re(CO)4(S2CNMe2). The dimer has unexpected stereochemistry; the open cubane structure appears stabilized by non-bonding interactions between opposing sulfur and dithiocarbamate central carbon atoms. Compound 3 was generated; to our knowledge this type of reaction where both photoproducts are captured in a single product has not been observed.
4. K. W. Lee and T. L. Brown, Inorg. Chem., 1987, 26, 1852-1856.
Copyright © 2014 American Chemical Society