58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI352119-DNI3: Acylation of Arenes via Catalytic C-H Bond Functionalization and Aerobic Alcohol Oxidation
Marion H. Emmert, PhD, Worcester Polytechnic Institute
1. OVERVIEW
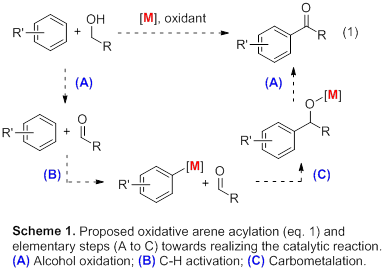
Despite the great advances in green chemistry in the last decades, many chemicals are still produced with low atom economies. Examples include arylketones, which are important bulk chemicals and substructures of bioactive compounds [[1]-[2][3]]. In order to create sustainable syntheses for arylketones, we have proposed to investigate a novel catalytic cycle (Scheme 1), consisting of (A) aerobic alcohol oxidations, (B) C-H activation, and (C) aldehyde carbometalation. Our progress towards gaining insight into the structure-activity relationships of catalysts for the individual steps is described in this technical report.
2. SCIENTIFIC REPORT
2.1 Ir catalyzed aerobic alcohol oxidations.
As the C-H activation step in the proposed mechanisms is well established with various catalyst systems [[4]-[5][6]], we decided to initially focus on aerobic alcohol oxidations in air. Even though active Cp*Ir catalysts under 1 atm O2 are known [[7],[8]], no systematic studies of ligands, additives, solvent effects, or O2 partial pressures have been reported.
Our investigations (Scheme 2) show that yields of arylketones as oxidized products with Cp*Ir catalysts are highly dependent on the solvent and the X‑type and L-type ligands. In contrast to literature reports on other Ir catalyzed alcohol oxidations [7], the presence of a base is not required to obtain turnover in our systems. Water, a possible by-product of aerobic arene acylation, improves the product yield. In contrast, the catalytic activity is greatly reduced, when air is exchanged for N2; therefore, we propose an oxidative pathway and not a dehydrogenative pathway [[9]] for the studied Cp*Ir systems.
The low yields (~70%) can be rationalized by the formation of catalytically inactive Ir-H complexes as decomposition products, which have been detected in the reaction mixture (1H NMR: d = -15 ppm). Analogous complexes have been reported in similar oxidations [7]. Current efforts are focused on ligand and reaction design aimed at suppressing the formation of these decomposition products during catalysis.
2.2 Pd catalyzed aerobic alcohol oxidations.
We further investigated aerobic oxidations with Pd catalysts for which decomposition to Pd metal has historically also been a problem. Through ligand screening and reaction design, our studies have established the first stable Pd catalysts for air oxidations of alcohols that can be assembled in situ from commercial starting materials. The catalysts achieve high turnover numbers and show quantitative conversions for a wide scope of substrates. This study has been published in Tetrahedron.
2.3 Aldehyde carbometalations.
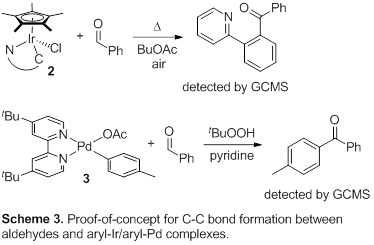
In subsequent studies, we investigated the C-C bond formation through aldehyde carbometalation, step (C) in the mechanistic hypothesis proposed above (Scheme 1). The Pd and Cp*Ir aryl complexes 2 and 3 were prepared according to literature procedures [5,[10]] and were reacted with benzaldehyde (Scheme 3). In both cases, the formation of the aryl ketone product was observed by GCMS in the presence of air or tBuOOH as oxidants. Current efforts focus on optimizing the yields of these reactions.
In conclusion, we have shown that aldehyde carbometalation and aerobic alcohol oxidations are feasible with both Cp*Ir and PdII complexes. The second year of the funding period will focus on completing these studies as well as on exploring structure-activity relationships in C-H activation catalysis through H/D exchange, as laid out in the original proposal.
3. IMPACT OF AWARD ON PI'S CAREER AND ON SUPPORTED STUDENTS
The ACS PRF award has benefited the development of the PI's research career and her research group in many ways. Because the award was received during the first year of the PI's appointment, the departmental recognition was considerable. The PI was then quickly able to hire her first externally funded researcher, a postdoc, with highly suitable qualifications. The postdoc is experienced in organometallic synthesis; he happily provides advice to younger students and has a great work ethic, which in turn benefits the whole research team.
Research performed under this grant has resulted in our first independent publication in Tetrahedron, and has provided data for 3 poster presentations by the PI (2 at Gordon Research Conferences; 1 at WPI), 1 poster presentation by the postdoc, and 1 poster presentation by an undergraduate researcher in the group. The PI has spoken about the work conducted under this grant in 2 invited lectures (Worcester State; University Munster, Germany). For the immediate future, one poster presentation by the postdoc at NERM 2013 and two invited lectures by the PI during the fall 2013 are scheduled, which will be used to disseminate research results more broadly. Some of the results have been used to apply for NSF funding through the CAREER mechanism in 2013.
The influence of the grant on students in the PI's laboratory has been substantial: The postdoc was able to collect experience in mentoring 2 undergraduate research volunteers and 1 senior thesis student. All 3 of these students will continue to work in the lab during the academic year 2013/14 in collaboration with the postdoc. Additionally, a self-funded German exchange student was able to complete a publication as a first author during his senior year, which made his graduate school application much more competitive.
[1] Albericio, F. Curr. Opin. Chem. Biol. 2004, 8 (3), 211-221.
[2] Bauer, K.; Garbe, D.; Surburg, H., Common fragrance and flavor materials. 4th Edition ed.; Wiley-VCH: Weinheim, 2001.
[3] Hwang, Y.-L.; Bedard, T. C., Ketones. In Kirk-Othmer Encyclopedia of Chemical Technology, Wiley: 2000.
[4] Gutierrez, M. A.; Newkome, G. R.; Selbin, J. J. Organomet. Chem. 1980, 202 (3), 341-350.
[5] Li, L.; Brennessel, W. W.; Jones, W. D. J. Am. Chem. Soc. 2008, 130 (37), 12414-12419.
[6] Yoshino, T.; Ikemoto, H.; Matsunaga, S.; Kanai, M. Angew. Chem. Int. Ed. 2013, 52 (8), 2207-2211.
[7] Jiang, B.; Feng, Y.; Ison, E. A. J. Am. Chem. Soc. 2008, 130 (44), 14462-14464.
[8] Kölle, U.; Fränzl, H., Oxidation of Alcohols by [Cp*Rh(ppy)(OH)]+. In Organometallic Chemistry and Catalysis, Kirchner, K.; Weissensteiner, W., Eds. Springer Vienna: 2001; pp 97-102.
[9] Kawahara, R.; Fujita, K.; Yamaguchi, R. Angew. Chem. Int. Ed. 2012, 51 (51), 12790-12794.
[10] Ball, N. D.; Sanford, M. S. J. Am. Chem. Soc. 2009, 131 (11), 3796-3797.
Copyright © 2014 American Chemical Society