58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND351178-ND3: The Role of Protons in Charge Transfer Reactions of Metal Oxide/Solution Interfaces
James M. Mayer, PhD, University of Washington
Our ACS-PRF New Direction grant has enabled us to start and pursue a number of projects exploring how protons modulate the interfacial reaction chemistry of metal oxide nanoparticles (NPs) and related materials. This award was our first outside funding for this direction, and the work supported by ACS-PRF has been the basis for subsequently funded proposals from NSF and DARPA, and an internal proposal within the Center for Enabling New Technologies Through Catalysis (CENTC), an NSF Phase II Center for Chemical Innovation.
The interfacial oxidation/reduction (redox) chemistry of metal oxide surfaces and other semiconductors is fundamental in a number of areas, from catalysis to fuel cells to aqueous geochemistry. We have focused primarily on nanoparticles of zinc oxide and titanium dioxide as models for oxide interfaces, because these oxides are very well studied and used in a variety of applications. Despite the enormous number of publications in this area, the critical role(s) of protons has not received much attention. The fact that our first paper supported by this award appeared in Science illustrates the impact of our studies in this direction.
Zinc oxide nanocrystals (NCs) have been an almost ideal material for these studies because they are very well characterized and they form stable reduced particles upon UV irradiation in the presence of alcohols and other reductants. We prepare these NCs following well-known routes starting with controlled hydrolysis in ethanol/DMSO followed by capping with dodecylamine. To keep track of protons, our studies have entirely been in the aprotic solvents toluene or 1:1 toluene/THF (for solubility of charged reagents).
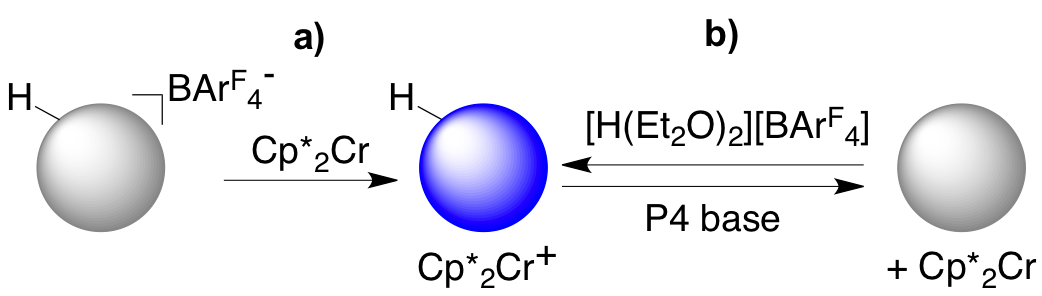
In this grant period, we reported that the capped ZnO NCs can be reduced with the strong chemical reductant decamethylcobaltocene (Cp*2Co) in toluene/THF solution, up to 1-3 electrons per NC. The optical and EPR spectra of these charged particles are essentially the same as the photochemically charged ones, even though these have Cp*2Co+ as the counterion rather than a proton. Protons can be added stoichiometrically to the NCs by either a photoreductionoxidation sequence or by addition of acid. The added protons facilitate the reduction of the ZnO NCs. In the presence of acid, NC reduction by Cp*2Co can be increased to over 15 electrons per NC. The weaker reductant Cp*2Cr only transfers electrons to ZnO NCs in the presence of protons, as shown in Scheme 1. Cp*2M+ counterions are much less effective than protons at stabilizing reduced NCs. With excess Cp*2Co or Cp*2Cr, the extent of reduction increases roughly linearly with the number of protons added. These studies represent a new method to stoichiometrically study the effect of each proton on the redox chemistry of NCs.
Scheme 1. Reduction of ZnO NCs by Cp*2Cr modulated by protons.
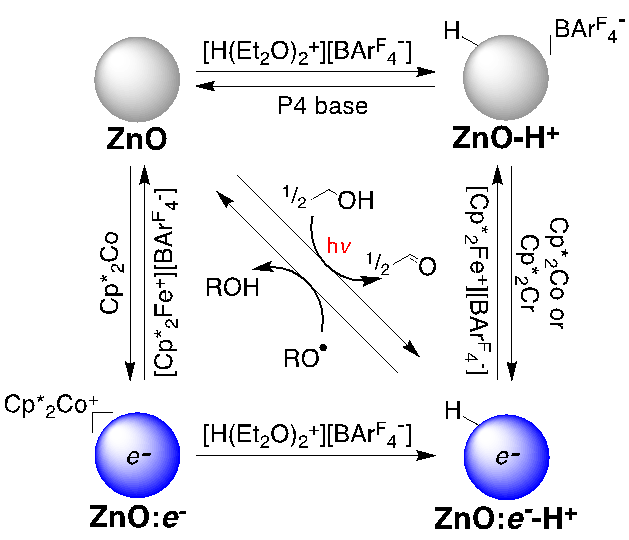
Scheme 2. PCET square scheme showing reactions that
interconvert charged and protonated ZnO NCs.
Current studies are exploring the influence of the dodecylamine (DDA) capping ligands on the properties of the ZnO NCs. Using primarily NMR spectroscopy, we have found the surfaces of ZnO NCs are incompletely covered with DDA. This contrasts with the typical picture of a complete monolayer of capping groups on ZnO NCs, and it provides an explanation for why capped NCs can participate in facile redox reactions. The surface coverage density of DDA ligands appears to modulate the rates of such reactions, from stopped-flow kinetic studies.
In parallel with the studies of ZnO NCs, we have been examining TiO2 nanoparticles (NPs) and related materials. In the last grant period, we have been examining uncapped TiO2 NPs in aqueous acid solution. We have shown that, contrary to a literature report, reduced version of these NPs do not convert N2 with NH3. We are, however, studying their participation in other interesting multielectron redox reactions. We have also started a collaboration with a group in Belgium that has prepared metal-organic-framework (MOF) materials with nodes of Ti8-oxide clusters. There are strong parallels between the PCET reactivity of molecular solution Ti8 clusters, Ti8-containing MOFs, and the TiO2 nanoparticles. Because studies of these different systems can reveal complementary aspects of the PCET chemistry, we are excited to be following these in parallel.
All of these experiments are building toward an understanding of how protons and electrons modulate and participate in the reactivity of the metal-oxide surface. We are excited that our new approach is providing interesting and valuable new insights in this important area.
Copyright © 2014 American Chemical Society