58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI151900-UNI1: Investigation of the Stabilization of Boron-Containing Cations by Azametallocene Donors: Applications to Reduction, Hydroboration and Borylation Reactions
Timothy J. Brunker, DPhil, Towson University
Since the start of the grant period we have made substantial progress on the first three of our specific aims.
Aim (i): Synthesis of a representative group of metal-bound heteroarene-borane complexes
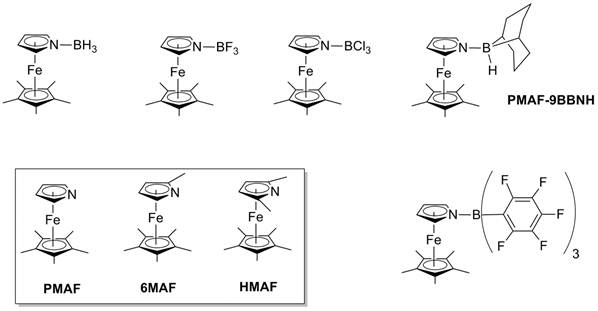
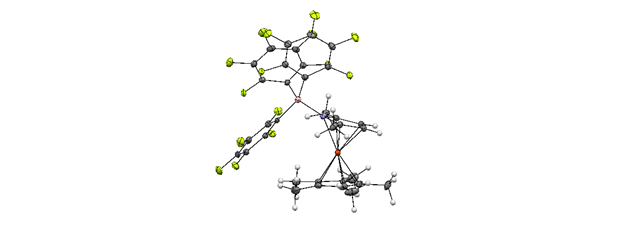
Our preliminary studies focused on 1',2',3',4',5'-pentamethylazaferrocene (PMAF) and to date we have synthesized BH3, BF3, 9-bora-bicyclo[3.3.1]nonane, BCl3 and B(C6F5)3 adducts of this azaferrocene. We have also synthesized both 1',2,2',3',4',5'-hexamethylazaferrocene (6MAF) and 1',2,2',3',4',5,5'-heptamethylazaferrocene (HMAF) which have one or two alpha-methyl groups on the pyrrolyl ring respectively. Both 6MAF and HMAF appear in the literature, although a preparation of HMAF has never been reported and only minimal characterization data presented. We have completely characterized this compound including an X-ray crystal structure. The same set of borane-adducts has been synthesized as for PMAF with these two azaferrocenes, with the exception of HMAF-9-bora-bicyclo[3.3.1]nonane which could not be isolated. This may be due to steric hinderance. Single crystal X-ray structures have been obtained in some cases, including PMAF-BCl3, PMAF-9-bora-bicyclo[3.3.1]nonane, HMAF-BF3 and HMAF-B(C6F5)3. The structure of HMAF-B(C6F5)3 reveals considerable distortion of the HMAF moiety from a regular sandwich structure to accommodate the bulky triarylborane. It is interesting to note that the N-B bond here is shorter than in the analogous lutidine-B(C6F5)3, despite the apparent greater 3-dimensional steric bulk of HMAF.
Figure 1: Azaferrocenes and borane adducts.
Figure 2: ORTEP plot of crystal structure of HMAF-B(C6F5)3
Aim (ii): Investigation of the formation of borenium ion species from these complexes
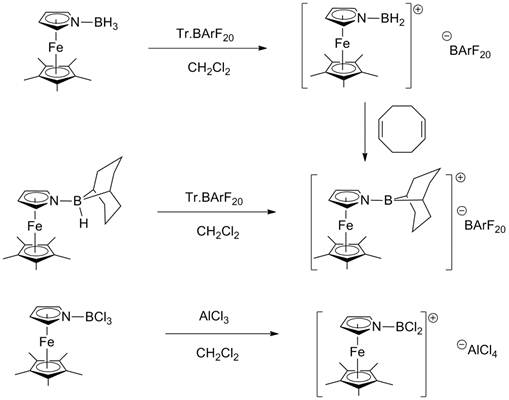
Hydride abstraction from PMAF-BH3 was previously shown to produce the borenium ion species [PMAF-BH2]+ as long as the trityl salt with [B(C6F5)4]- counterion was utilized. Use of the BF4- ion led to fluoride abstraction from the counterion. The analogous borenium ions of [6MAF-BH2]+ and [HMAF-BH2]+ have been observed in the same way: All are characterized by an 11B NMR peak at around 40 ppm. Attempts to isolate [PMAF-BH2]+ were unsuccessful, but the boronium species [(PMAF)2BH2]+ was characterized after addition of excess PMAF to the borenium ion. Similarly hydride abstraction from PMAF-9-bora-bicyclo[3.3.1]nonane gave [PMAF-9BBN]+: Again if the BF4- -containing trityl salt was used, the known 9-fluoro-9-bora-bicyclo[3.3.1]nonane was observed by 11B NMR as the product of fluoride abstraction. The 6MAF-9-bora-bicyclo[3.3.1]nonane adduct also forms the borenium ion [6MAF-9BBN]+. Both of these dialkylborenium ions are characterized by an 11B NMR peak at around 70 ppm. Attempts to trap [PMAF-9BBN]+ with another Lewis base (PMAF or N-methylimidazole (NMI)) have not been successful although if excess NMI was used, the boronium salt (NMI)2(9-BBN)+ could be isolated and characterized crystallographically. Reaction of PMAF-BCl3 with AlCl3 gave [PMAF-BCl2]+[AlCl4]- by chloride abstraction. This borenium cation was characterized crystallographically: Some bending of the exocyclic B atom towards Fe was observed along with the expected changes in geometry associated with rehybridization of the B from sp3 to sp2. DMAF and HMAF-BCl3 adducts behave similarly: All these –BCl2+ containing borenium ions are characterized by peaks at 35-40 ppm in 11B NMR. Some DFT calculations have been performed by my colleague Prof. Shuhua Ma on the geometry of [PMAF-BH2]+ which displays similar changes to this crystal structure. We have assessed the relative Lewis acidity of all these borenium ions in the PMAF series by the Gutmann-Beckett 31P NMR method utilizing Et3P=O as the reference Lewis base and compared them to B(C6F5)3. The Lewis acidity decreases in the order [PMAF-BH2]+ > [PMAF-9BBN]+ > [PMAF-BCl2]+ > B(C6F5)3.
Figure 4: Summary of borenium ion formation chemistry
Figure 3: ORTEP view of the crystal structure of [PMAF-BCl2]+.
Aim (iii): Survey of the reactivity of both –borane and –borenium complexes with respect to reduction, hydroboration and borylation chemistry
Reaction of [PMAF-BH2]+ with 1,5-cyclooctadiene gives [PMAF-9BBN]+ which was confirmed by NMR spectroscopy and the independent synthesis of the product borenium ion as described above. [6MAF-BH2]+ behaves similarly also to give the doubly hydroborated product. We are beginning experiments with isoprene. With other alkenes (β-methyl styrene and 1-octene) reaction with [PMAF-BH2]+ gives a mixture of mono- and dialklylated boranes after quenching with methanol, as evidenced by 11B NMR. Work will continue to assess the regioselectivity of the hydroboration in these cases. Despite efforts to effect aromatic borylation with several substrates with [PMAF-9BBN]+, none of the desired reactivity was observed. We have also briefly examined the potential of [6MAF-9BBN]+ and [PMAF-9BBN]+ to effect hydrosilylation of acetophenone with triethylsilane. Again no reactivity was observed. In studies arising from this, we did notice that 6MAF-BCl3 reacts with triethylsilane to cleanly give 6MAF-BCl2H: For, as yet, unknown reasons the analogous reactions with PMAF and HMAF-BCl3 do not proceed efficiently. Although not originally proposed, we have also briefly examined whether azaferrocene-B(C6F5)3 adducts display frustrated Lewis Acid-Base behavior by dissolving the adducts in toluene and stirring under an atmosphere of H2. No evidence of heterolytic H-H bond breaking has been observed. For HMAF-B(C6F5)3 this is consistent with the crystal structure that displays a relatively short B-N bond and solution NMR studies that show no evidence of borane dissociation, but restricted rotation around the N-B and B-C bonds.
Aim (iv): Synthesis of a resolved, chiral azaferrocene, its -borane and –borenium species and investigation of asymmetric reactions
Our studies on 6MAF, which have so far been conducted using only the racemic azaferrocene, will direct future studies using chiral, resolved azaferrocenes. In particular, we will examine asymmetric hydroboration with resolved [6MAF-BH2]+. We may derivatize 6MAF and HMAF to access even more bulky azaferrocenes either as racemic or enantiopure compounds. These would be surveyed for frustrated Lewis acid/base behavior also.
Impact of research
To date 3 undergraduate student researchers have worked on this project for credit as well as receiving summer research stipends for full time research. None of these students have yet graduated from Towson. We currently have a manuscript close to submission outlining the PMAF-borenium ion chemistry and we have plans to publish the HMAF and 6MAF borane chemistry shortly thereafter. The PI has made two conference presentations based on the research funded by this proposal. The PI undergoes review for promotion and tenure this year and this grant and the research described herein formed a significant component of the scholarship package.
Copyright © 2014 American Chemical Society