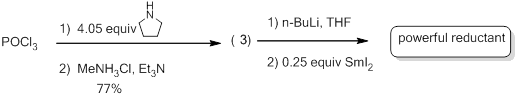58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR152067-UR1: The Characterization and Synthetic Utility of Complexes Made from Samarium Diiodide and Anionic Phosphoramides
Chriss E. McDonald, PhD, Lycoming College
Samarium diiodide is one of the most useful reagents employed by organic chemists. The use of this reagent is greatly enhanced by the addition of electron rich cosolvents. HMPA (1) is the most common cosolvent to be employed and the majority of modern SmI2 chemistry is accomplished with this cosolvent. Unfortunately HMPA is a mutagen which has lead to an extended search for suitable alternatives. The goals of this work are to find SmI2 activators which are both safer and more effective. We have recently reported that TPPA (2) is approximately an order of magnitude better at activating SmI2 at two of its key synthetic processes, reduction of carbon-halogen bonds and reduction of ketones. Our most recent work involves the use of deprotonatable phosphoramides such as DPMPA (3). It is thought that the conjugate base of 3 will deliver even more electron density to the metal center and produce a more reactive complex.
Compound 3 can be synthesized in multi-gram quantities via a two-step procedure using POCl3. Upon treatment of a THF solution of DPMPA with BuLi and exposure to SmI2, a deep brown (not violet) solution with powerful reductive capabilities is obtained.
The reduction of the reluctant radical precursor 1-chlorodecane was used as an assay for the relative reactivities of various SmI2/activator complexes. As can be seen in Table 1, SmI2/DPMPA- complexes of ratios 1:2, 1:3, and 1:4 are dramatically more reactive than other synthetically useful SmI2 complexes. Neutral phosphoramides (HMPA, diHMPA, TPPA, DPMPA) and SmI2/H2O/Et3N produce yields of 0-1% of decane under these very mild and dilute conditions. By contrast, the complex SmI2/4 DPMPA- affords a 90% yield of decane after one minute.
Table1. The Reduction of 1-Chlorodecane with SmI2 and Additives
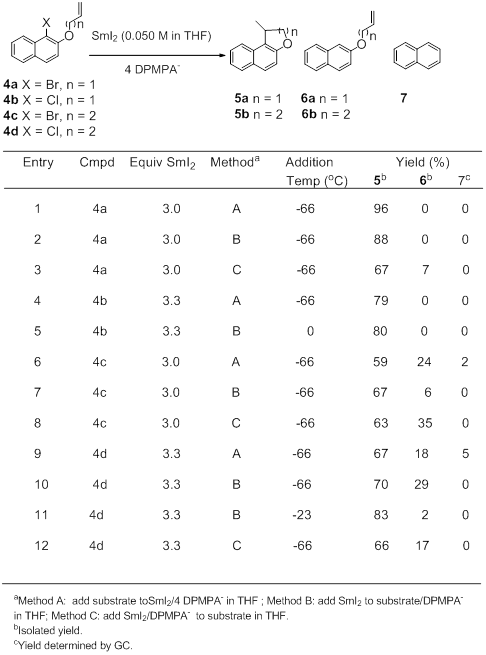
The 4:1 complex of DPMPA- and SmI2 has been used to reductively cyclize a group of haloalkenylnaphthalenes 4a-d as shown in Table 2. It has been determined that the best approach for these cyclizations involves the slow addition of SmI2 to a cold THF solution of substrate plus DPMPA- (Method B). These conditions minimize formation of the reduced uncyclized product 5 and naphthalene. The optimal temperature for this addition depends on the radical precursor. The more reactive bromides react smoothly at a starting temperature of -66oC. The less reactive chlorides work better at warmer temperatures. Cyclization of chlorobutenyl substrate 4d works best at a starting temperature of -23oC.
We have also characterized SmI2/ DPMPA- complexes by visible spectroscopy and cyclic voltammetry. Samarium diiodide in THF has absorption maxima at 553 and 621 nm and is blue in color. Neutral phosphoramides such as HMPA or TPPA produce a violet solution with a broad band centered at 540 nm. Addition of four equivalents of DPMPA- to SmI2 affords a brown solution with an absorption maximum at 405 nm. Cyclic voltammetry was used to attempt to quantify the reducing power of SmI2/ DPMPA- complexes. Samarium diiodide in THF has a standard potential of -1.33 V vs. Ag/AgNO3. Addition of two equivalents of DPMPA- to SmI2/THF affords a solution with a standard potential of -1.93 V, very close to the value of SmI2/4 HMPA (-2.07 V) and the more reactive SmI2/4 TPPA (-1.94 V). Instrumental limitations prevent the measurement of standard potential for SmI2/3 DPMPA- and SmI2/4 DPMPA- .
Table 2. The Reductive Cyclization of Haloalkenyl Naphthalenes.
Current work in this laboratory focuses on the use of other reluctant radical precursors, as well as the development of new SmI2 activators.
Copyright © 2014 American Chemical Society