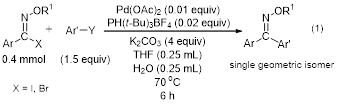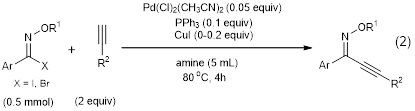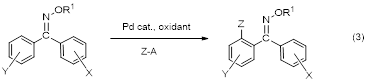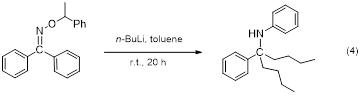58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR150612-UR1: New Synthetic Methodology to Prepare Imine Derivatives in Single, Predictable Geometric Configurations
Debra D. Dolliver, Southeastern Louisiana University
Progress toward the major goals outlined in this proposal for the scholastic year summer 2012 to fall 2013 are given below.
Progress toward Goal A: Establish a robust synthetic protocol to reliably synthesize single E or Z isomers of oxime ethers using a variety of imidoyl iodides and trifluoroborate salts
During the past year of the grant, the PI and the undergraduate students working under her direction have completed the optimization of the palladium catalyzed coupling of N-alkoxyimidoyl iodides and bromides with trifluoroborate salt and boronic acid coupling partners, and this work has been published.1 The optimized conditions for the Suzuki coupling protocol are shown in equation 1.
These reactions resulted in very high yields of the diaryl oxime ether with complete retention of the geometry around the C=N bond. The reaction was demonstrated to work well with a wide variety of substituents on the aromatic rings. This method provides an efficient route to synthesize single E or Z isomers of diaryl ketoxime ethers.
In addition, the PI and her students also completed a study of the coupling of N-alkoxyimidoyl iodide and bromide compounds in Sonogashira coupling reactions and Negishi coupling reactions, and these are also reported in the same paper.1 The optimized Sonogashira reaction was also found to be high yielding and tolerant of several different functional groups (equation 2).
The Negishi reaction persisted in giving an aromatic nitrile byproduct, thought to result from zinc-assisted ionization of the halide on the imidoyl halide, and the yield of the coupling product from this reaction was only moderate.
The success of this project in supplying a route to single geometric isomers of diaryl oxime ethers opened up the opportunity to explore palladium-catalyzed C-H activation/functionalization at the ortho position (equation 3). Work is currently underway on routes to selectively ortho brominate and ortho alkoxylate only one ring of these diaryl oxime ethers of single E or Z geometry. These reactions have been optimized and the geometry of the product has been determined in some cases by X-ray crystallography. This work will be completed in this upcoming year of the grant and will be submitted for publication in 2014.
Progress toward Goal B: Investigate the above coupling technique with N-alkoxyimidoyl pseudohalides
The Suzuki coupling reactions were attempted with the N-alkoxyimidoyl tosylates [ArC(OTs)=NOR]. This reaction yielded no coupling products. Because of the unreactivity of the tosylates in these reactions, further work in this area has been put on hold to pursue other successful lines of research at this time.
Progress toward Goal C: Investigate the coupling technique with other N-substituted imines
Work is currently underway studying the feasibility of using hydrazonyl bromides [R1C(Br)=N-NR2R3] successfully in palladium-catalyzed coupling reactions. One of the undergraduates working with the PI is currently synthesizing a series of hydrazonyl bromides with which to perform the palladium-catalyzed coupling trials. It is planned that the initial investigation into both the Suzuki and Sonogashira coupling reactions of the hydrazonyl bromides will be performed in the fall of 2013. Particular attention will be paid to the E/Z stereochemical outcome of these reactions. If these reactions yield some of the coupling products, optimization of the reaction conditions will be carried into the spring of 2014, and a full investigation of the stereochemistry of the resulting product will be undertaken.
Progress toward Goal D: Utilize the established coupling reaction to make biologically-active compounds
In the summer of 2013, one of the undergraduates working with the PI explored the reactions of various organometallic compounds with diaryl oxime ethers. For these initial studies, the O-1-phenethyl oxime of benzophenone was used as a model compound. It was chosen as a model because there is a precedent for using single enantiomers of O-1-phenethyl oxime of benzaldehyde to bias facial selectivity in nucleophilic attack by an organometallic.2 Disappointingly, reaction of n-BuLi with the oxime ether of benzophenone required almost room temperature conditions to facilitate nucleophilic attack. In addition, after attack of the butyl group, the molecule underwent an aromatic migration and loss of alkoxide to form an N-phenyl imine which underwent attack by a second butyl group, making the secondary amine PhC(Bu)2NHPh (equation 4). Thinking that exchanging the oxophilic lithium counterion in the organometallic for a more azaphilic one might diminish the apparent nitrene formation and the migration of the phenyl group, the reaction was tried with an allylbismuth reagent/metallic zinc complex known to be an effective allylation medium for aldehydes.3 Unfortunately, reaction of this complex with the diaryl oxime ether resulted only in recovery of starting material. Other organometallic compounds are currently being explored as possible reagents to make a new carbon-carbon bond to the carbon of the C=N bond in these compounds.
Progress toward the general goal of providing solid research training to undergraduates:
The funds from the PRF grant have allowed the PI to productively engage undergraduate students in research that has produced publishable results. One of the students financially supported by the PRF grant was a co-author on a peer-reviewed publication.1 This same student will be graduating in December, 2013, and he intends to attend graduate school in chemistry upon graduation. The other students who have been supported by the grant have not yet graduated, but they are all considering pursuing careers in chemistry.
References:
- Dolliver, D. D.; Bhattarai, B. T.; Pandey, A.; Lanier, M. L.; Bordelon, A. S.; Adhikari, S.; Dinser, J. A.; Flowers, P. F.; Wills, V. S.; Schneider, C. S.; Shaughnessy, K. H.; Moore, J. N.; Raders, S. M.; Snowden, T. S.; McKim, A. S.; Fronczek, F. R.; J. Org. Chem., 2013, 78 (8), 3676-3687.
2. Gallagher, P. T.; Hunt, J. C. A.; Lightfoot, A.P.; Moody, C.J. J. Chem. Soc., Perkin Trans. 1, 1997, 2633-2637.
3. Wada, M.; Ohki, H.; Akiba, K.; Tetrahedron Lett. 1986, 27 (39), 4771-4774.
Copyright © 2014 American Chemical Society