58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI351754-DNI3: Prodigiosin-Based Platforms in Metalloradical Complexes for Dioxygen Activation and Oxidative Catalysis
Elisa Tomat, PhD, University of Arizona
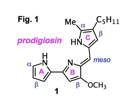
The tripyrrolic natural product prodigiosin (1, Fig. 1 indicating the nomenclature of the three pyrrole rings as well as the positions commonly referred as α, β or meso positions) is the parent compound of the large family of pyrrolyldipyrrins, which are currently undergoing intense scrutiny in medicinal chemistry because of their biological activity in a variety of settings. Interestingly, prodigiosin 1 cleaves double-stranded DNA in the presence of O2 and Cu(II) cations without the need of an added reductant.
The potential of pyrrolyldipyrrins as chelating units for transition metals, however, remains largely unfulfilled. In addition, the prodigiosin-induced oxidative cleavage of nucleic acids is consistent with formation of p-radical cations on the pyrrolic scaffold and metal-assisted activation of dioxygen. Nevertheless, the chemistry of this family of ligands remains largely unknown and direct connections between chemical properties and catalytic potential in dioxygen activation have not been established.
a) Synthesis of pyrrolyldipyrrins
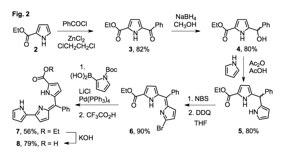
We sought to build a pyrrolyldipyrrin of higher metal binding affinity when compared to natural systems. We reasoned that such a system would allow investigation of the properties of metal-bound pyrrolyldipyrrins. Specifically, in order to increase the acidity of the pyrrolic N-H protons, we introduced two electron-withdrawing groups in the first-generation fragment 7: (i) a phenyl group in the meso-type position and (ii) an ethyl ester group on the C-ring, which could contribute to metal coordination with an oxygen donor group. In addition, with a stabilized ¹ system and a blocked site of potential oxidation (i.e., pyrrolyc α carbon on the C ring), we anticipated that such construct would be less prone to oxidative degradation. Both the ethyl ester 7 and the carboxylate 8 were isolated and characterized. The relative orientation of the pyrrolic rings in 7, which was also identified as most stable for unsubstituted pyrrolyldipyrrins, was confirmed by 2D NMR experiments. When compared to the other available syntheses of meso-aryl pyrrolyldipyrrins, our synthetic route is higher yielding and more modular, thus more amenable to derivatization and preparation of analogues.
Cyclic voltammetry experiments on compound 7 revealed more positive anodic peak potentials (+0.51 V and +0.98 V vs Fc/Fc+ in acetonitrile) for the irreversible oxidation events that were previously recorded for the naturally occurring prodigiosin 1 (+0.06 V and +0.51 V vs Fc/Fc+ in acetonitrile). In addition, the UV-visible absorption spectrum of 7 presented a shift of the visible absorption band to shorter wavelengths, consistent with stabilization of the HOMO for the ¹-¹* transition assigned to the band. These comparative data are in agreement with expected effects of two added electron-withdrawing groups and demonstrate the tunability of conjugated ¹ systems in pyrrolyldipyrrins.
In summary, pyrrolyldipyrrin 7 is a more robust platform for metal coordination when compared to known fragments in its class, and a useful starting point for an evaluation of the factors governing their coordination abilities and redox behavior.
b) Study of metal coordination
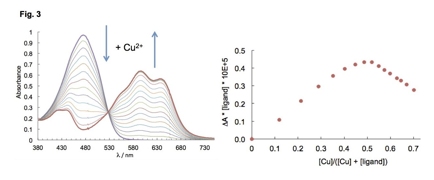
We investigated the interaction of our new pyrrolyldipyrrin 7, which is more stable to oxidative degradation relative to natural prodigiosin, with the redox-active Cu(II) ion. Binding equilibria in the presence of Cu(OAc)2 in THF solutions were monitored by UV-visible spectrophotometry (Fig. 3, left, [7] = 36 μM, [Cu(OAc)2] = 0.0-2.5 equiv.). Consistent with mass spectrometry data, ligand 7 forms a single copper complex with 1:1 binding stoichiometry as confirmed by Job's analysis (Fig. 3, right). Unlike reactions with previously reported pyrrolyldipyrrins, addition of base is not required and oxidative degradation is not observed by mass spectrometry analysis. The copper coordination mode of compound 7 will be further investigated both in solution and in the solid state by EPR spectroscopy and X-ray diffraction analysis, respectively.
Although pyrrolyldipyrrin ethyl ester 7 forms a 1:1 complex with Cu(II) ions in THF, a 2:1 zinc complex was isolated for natural prodigiosin 1 lacking a coordinating group on the C ring. The tripyrrolic scaffold can therefore act as a bidentate or tridentate ligand, thus leading to tetrahedral or pseudo-planar geometries. Both the stoichiometry and binding modes of the prepared ligands will be investigated upon insertion of manganese, iron, copper and zinc. Notably, this investigation will seek to document the first study of pyrrolyldipyrrin coordination of iron - the most abundant transition metal in living systems. The potential for dioxygen activation chemistry and redox catalysis will be evaluated for all complexes.
The findings summarized in this report will be submitted shortly as a first publication on this new project. Funding from the American Chemical Society Petroleum Research Fund is critical in this takeoff phase of the project and we believe that the collected data will form a solid basis for our future applications seeking federal funding for our research program. In addition, this funding has allowed training of one graduate and one undergraduate student at their first research experience and now making good progress towards their respective degrees.
Copyright © 2014 American Chemical Society