58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI350974-DNI3: Synthesis of N-Heterocyclic Carbene-Dithiolate Pincer Ligands and their Transition Metal Complexes: Investigation of Reactivity and Ligand Redox Behavior
Christine M. Thomas, PhD, Brandeis University
Summary of Proposed Work
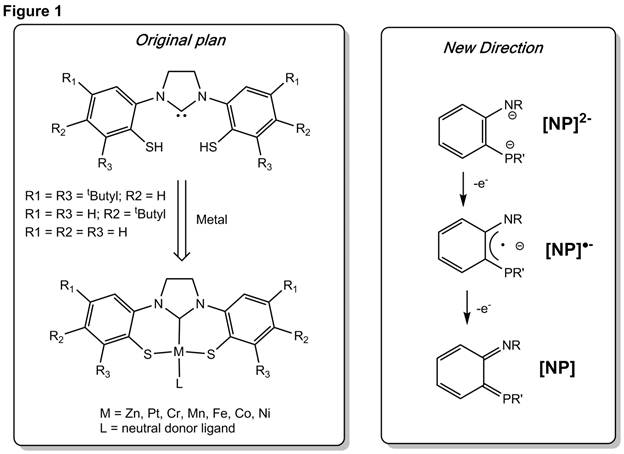
The proposed project involved the investigation of a series of N-heterocyclic carbene-containing dithiolate pincer ligands and their coordination chemistry with a series of transition metals (Figure 1). Group 10 metal complexes featuring the parent ligand (all R groups in Figure 1 = H) had been previously synthesized by Sellmann and coworkers via a one-pot route, and we had hoped to expand the coordination chemistry of this ligand variety to a number of different metals. This proved more challenging than originally anticipated and expansion to transition metal complexes was not straightforward. Thus, during this funding year we turned our attention to a new series of ligand derivatives with the ultimate goal of uncovering redox activity in ligands featuring P donor atoms. While a number of aryl-based bidentate redox active ligands have been investigated with O, S, and N donors, there are currently no examples of similar P-based ligands: [NP]2- (Figure 1). Thus, we have begun to investigate aryl-linked phosphide/amido ligands and their coordination chemistry.
Ligand Synthesis
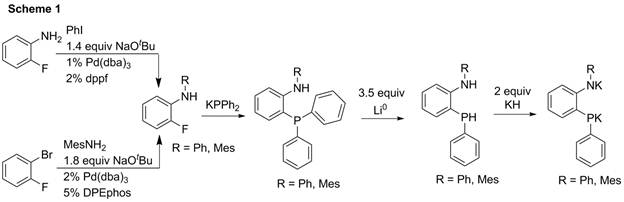
The proposed [ArNP]2- ligands (with phenyl substituents on phosphorus and aryl substituents on nitrogen to help stabilize potential ligand radicals) could be synthesized in three steps in high overall yield (50-70%), as shown in Scheme 1. Both the amine and phosphine moieties could be easily deprotonated with KH to generate the amidophosphido ligand.
Complexes of Group 10 Metals
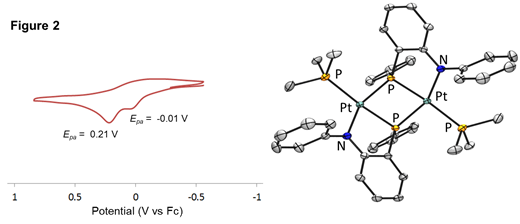
Metallation of the [ArNP]2- was then carried out using a range of Group 10 L2MCl2 precursors (M = Ni, Pd, Pt; L = PMe3, PPh3, COD, etc), and in all cases, phosphide-bridged dimers {[NP]ML}2 were isolated. A representative crystal structure of the Pt dimer {[PhNP]Pt(PMe3)}2 is shown in Figure 2 (right). Cyclic voltammograms of all isolated {[NP]ML}2 complexes were collected to determine whether any oxidation chemistry (ligand OR metal-based) was accessible at relatively mild potentials. As shown in Figure 2 (left) the representative {[PhNP]Pt(PMe3)}2 complex features two irreversible oxidative processes in its cyclic voltammogram, which are tentatively assigned to each half of the complex (the oxidation of one metal-ligand unit presumably shifts the oxidation of the second metal-ligand unit to ~0.2 V more positive potential). To determine whether these oxidative processes are metal- or ligand-centered, bulk chemical oxidation of {[PhNP]Pt(PMe3)}2 was carried out using Ag+, leading to an immediate color change to intense inky blue. Although this oxidized species has not been isolated or structurally characterized, we note that “blue” is a common color for purported Pt(III) species in the literature. Attempts are ongoing to isolate this “{[PhNP]Pt(PMe3)}2+” oxidation product, and other oxidized species featuring Group 10 metals and the phosphidoamido ligands, as structural and spectroscopic information will be needed to determine the extent of ligand involvement in the oxidation process.
Coordination to Copper: Evidence for Ligand Redox Activity
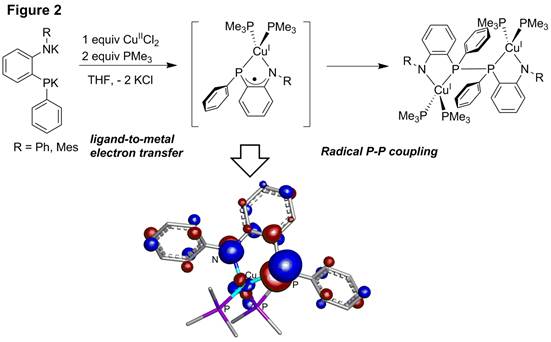
Treatment of the [ArNP]2- ligands with the Cu(II) salt CuCl2 in the presence of PMe3 results in formation of a dimeric Cu(I) product in which a phosphorus-phosphorus linkage has been formed (Figure 2). This product provides direct evidence for redox activity, since Cu(II) has ultimately been reduced to Cu(I) via oxidation of the amidophosphido ligand and formation of a P-P bond through radical dimerization. The proposed intermediate of this metallation reaction involves a ligand-centered radical formed via oxidation of the [ArNP]2- ligand by Cu(II). The existence of this [ArNP]●-/Cu(I) intermediate was probed using computational methods, and this intermediate was found to have very little radical character on the Cu center (consistent with Cu(I)) and significant radical character on the phosphorus atom, explaining this intermediate's propensity for radical P-P coupling.
New Ligand Derivatives
While the aforementioned Cu chemistry provides a convincing proof-of-principle (i.e. that redox-active ligands with P donor atoms can behave similarly to those with O, N, and S donor atoms), perhaps more interesting chemistry may ensue if such radical dimerization is presented. Thus, new ligand derivatives featuring more sterically encumbering substituents on the phosphide donor are currently under synthetic development (Figure 3).
An undergraduate in the laboratory has also developed a new ligand with an alkyl substituent on nitrogen and has been examining the coordination chemistry of this ligand to investigate its redox behavior. We have found that, as expected, the more electron-rich isopropyl substituent on the [ArNP]H2 ligand precursor renders the amine less acidic, necessitating a stronger base. For example, treatment of the [ArNP]H2 precursor with KH in the presence of NiCl2(PPh3)2 leads to a trimetallic nickel complex with protonated amines, while carrying out the same reaction with a stronger base such as benzylpotassium leads to a structurally similar Ni trimer with deprotonated amide donors (Figure 3). The coordination chemistry and redox activity of this ligand is currently under investigation.
Impact of the Research
While the initial goals of this research project were not attained, new progress has been made towards a new type of donor (phosphide) in redox-active ligands. This fundamental information will provide insight into the development of new catalysts for multielectron redox processes such as those involved in the activation of s bonds in alternative fuels.
The research has, thus far, impacted my career by allowing me to explore a new and interesting fundamental research direction. We were able to quickly establish a research area that was not worth pursuing and move on to a new, but related, direction. This sort of exploratory freedom is extremely important for a new PI as they establish their niche in chemical research.
The postdoctoral fellow, graduate student, and undergraduate working on this project have learned a lot through the successes and failures of this project. They have learned a lot about synthetic inorganic chemistry, including characterization methods such as NMR spectroscopy and X-ray crystallography, and about chemical redox processes and how they can be probed using cyclic voltammetry, spectroscopic methods, and key control experiments.
Copyright © 2014 American Chemical Society