58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND752135-ND7: Biomimetic Functionalized IPNs - Harnessing the Power of Phase Interactions
LaShanda TJ Korley, Case Western Reserve University
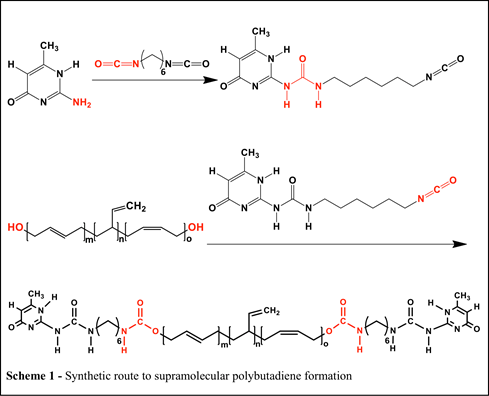
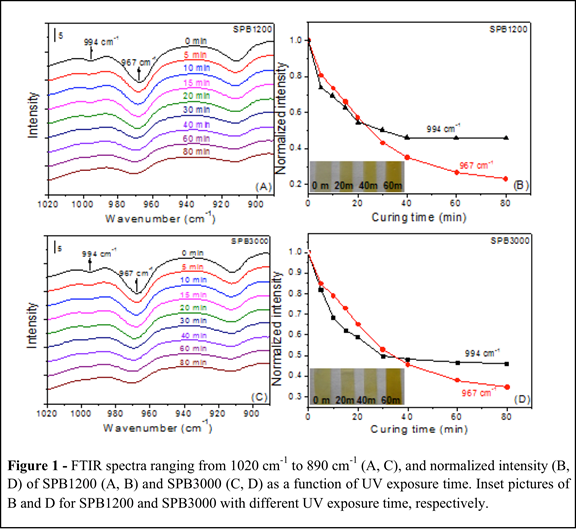
(2) Mechanical response of photocurable supramolecular elastomers
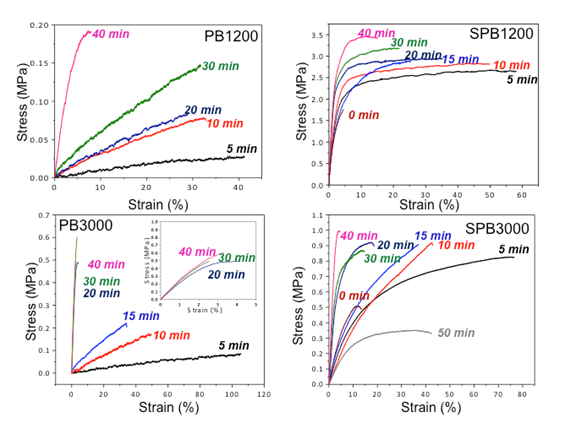
Figure 2 - Tensile behavior of cured SPB1200 and SPB 3000 compared to PB1200 and PB3000
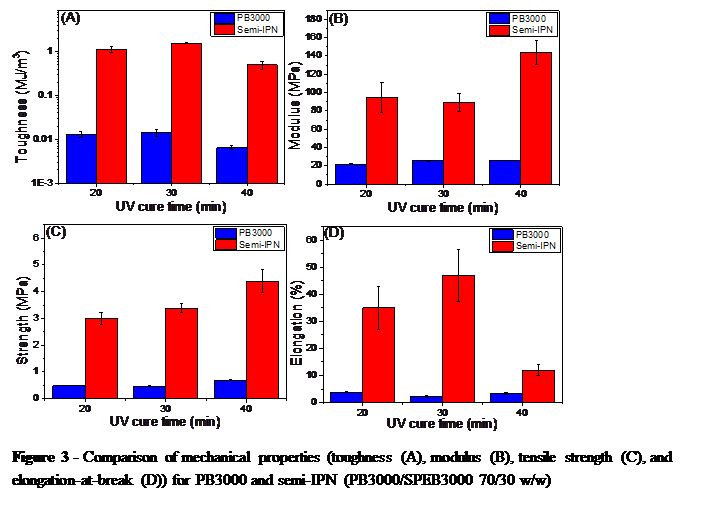
Figure 3 - Comparison of mechanical properties (toughness (A), modulus (B), tensile strength (C), and elongation-at-break (D)) for PB3000 and semi-IPN (PB3000/SPEB3000 70/30 w/w)
Copyright © 2014 American Chemical Society