58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR151036-UR1: Applications of Iron(III) Compounds as Catalysts in Organic Synthesis
Ram S. Mohan, Illinois Wesleyan University
Project # 1
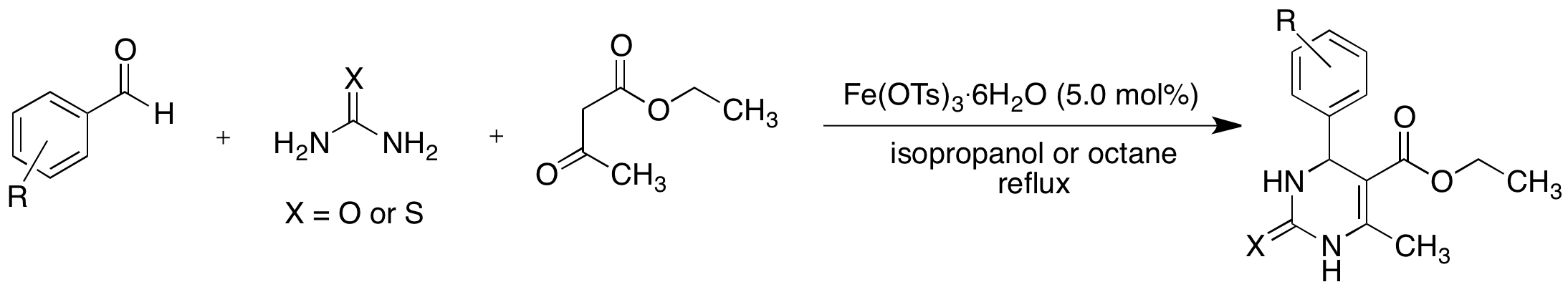
Iron(III) Tosylate Catalyzed Synthesis of 3, 4-Dihydropyrimidin-2(1H)-ones/thiones via The Biginelli Reaction
The synthesis of dihydropyrimidinones and dihydropyrimidine thiones has attracted interest due to their biological activities. A common method for their synthesis is the Biginelli reaction, which is a one-pot condensation of an aryl aldehyde, urea (or thiourea), and ethyl acetoacetate. The Biginelli reaction is typically catalyzed by a Brznsted or Lewis acid. However, many of these catalysts such as BF3∙Et2O and AlCl3 are corrosive and/or toxic. Our continued interest in environmentally friendly organic synthesis prompted us to investigate the utility of iron(III) tosylate as a catalyst for the Biginelli reaction (Scheme 1).
Scheme 1
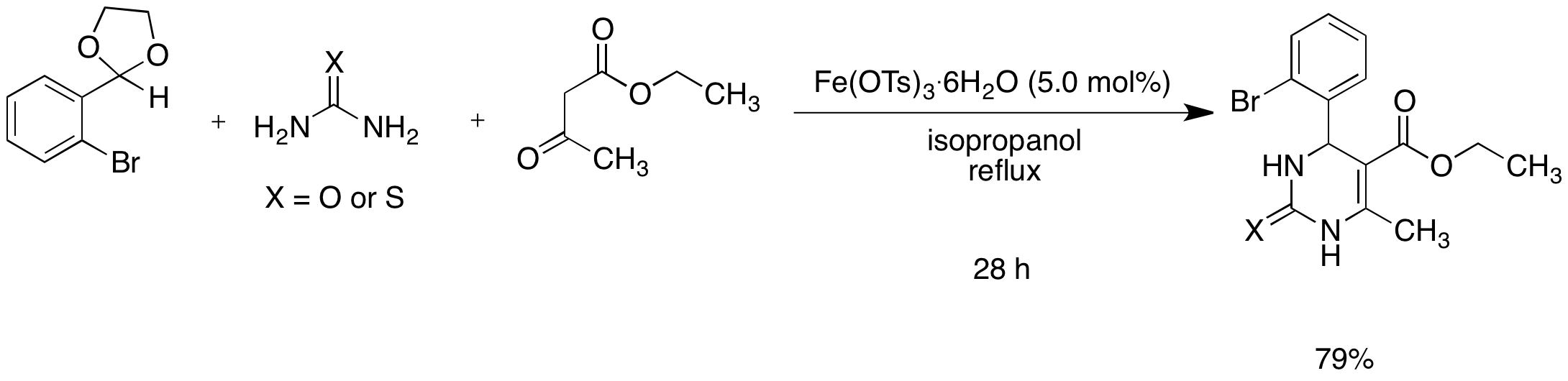
The use of acetals in the Biginelli reaction is also reported. Iron(III) tosylate is an attractive catalyst for the Biginelli reaction because of its low cost, low toxicity, and ease of handling. The observation that a solution of iron(III) tosylate in water is acidic (pH ~ 2) suggests that the p-TsOH might be an active catalyst in this reaction, though the role of Fe3+ as a Lewis acid cannot be ruled out. The use of Fe(OTs)3 is still preferable to p-TsOH because the latter compound is highly toxic and poses a health hazard. Although the reaction worked with lower catalyst loading, the best results were obtained with the use of 5.0 mol% catalyst. Both isopropanol and octane worked as solvents. Although octane is less environmentally friendly than isopropanol, it was easily recovered using a rotary evaporator or by decantation and recycled. Both procedures avoid an aqueous work-up and hence large aqueous waste streams were avoided, thus adding to the green aspect of the methodology. We have previously shown that iron(III) tosylate is an efficient catalyst for the deprotection of acetals. Hence we attempted the Biginelli reaction with a range of acetals and found promising results. To the best of our knowledge this is the first example of the use of acetals in the Biginelli reaction (Scheme 2). Since acetals are frequently used to protect aldehydes, this method allows the direct synthesis of useful heterocycles from acetals without the need for an additional deprotection step.
Scheme 2
Project # 2
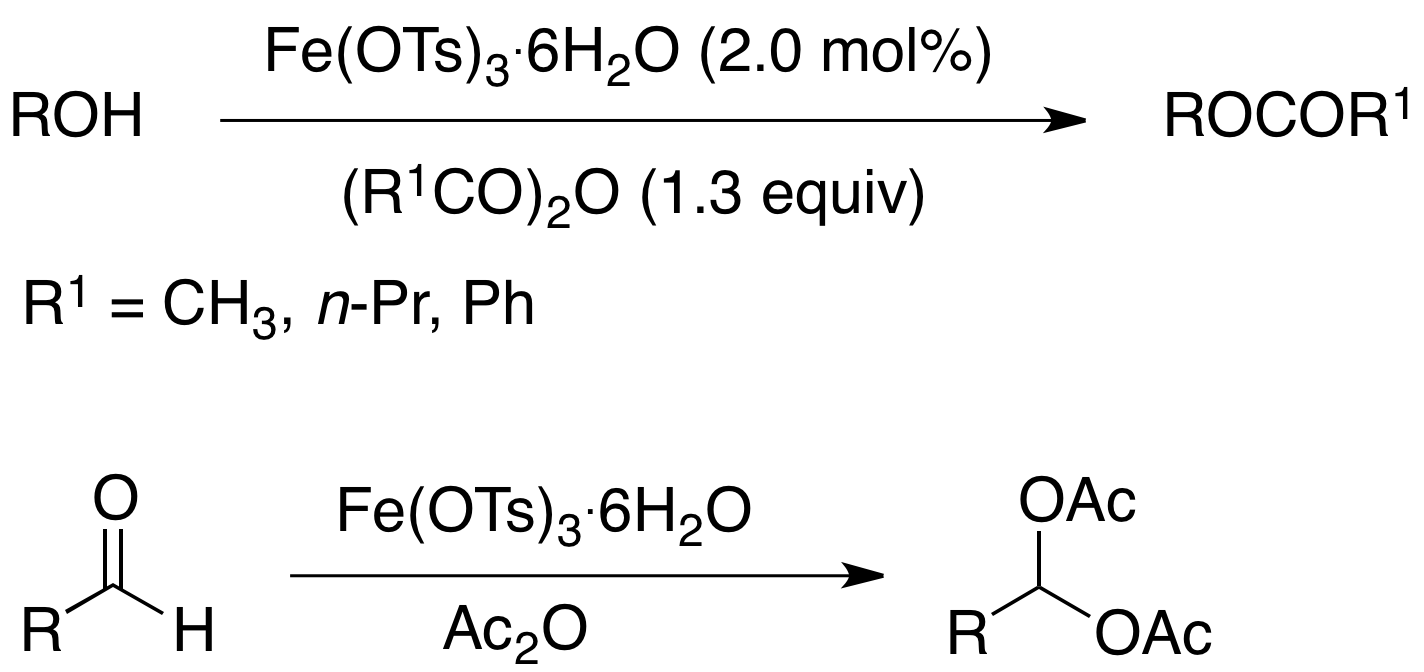
Iron(III) Tosylate Catalyzed Acylation of Alcohols, Phenols and Aldehydes.
Iron(III) p-toluenesulfonate (tosylate) is an efficient catalyst for acetylation of alcohols, phenols, and aldehydes. The acetylation of 1° and 2° alcohols, diols, and phenols proceeded smoothly with 2.0 mol % of catalyst. However, the reaction worked only with a few 3° alcohols. The methodology was also applicable to the synthesis of a few benzoate esters but required the use of 5.0 mol% catalyst. Aldehydes could also be converted to the corresponding 1,1-diesters (acylals) under the reaction conditions. Iron(III) tosylate is an inexpensive and easy to handle, commercially available catalyst.
When acetic anhydride was used as the acylating agent the reaction could be carried out under solvent-free conditions. With allylic alcohols the use of solvent (CH3CN) gave fewer side products. Solvent was also necessary when the acylating agent (benzoic anhydride) was a solid, the reaction mixture solidified without solvent, or if the starting alcohol was poorly soluble in acetic anhydride. In most cases, the crude product was found to be ³ 98% pure by 1H and 13C NMR spectroscopy and further purification was deemed unnecessary. Although the methodology was not broadly applicable to tertiary alcohols, we were able to successfully acetylate some 3° alcohols. For example, 1-ethynylcyclohexanol gave a moderate yield of the corresponding acetate. When 1-methylcyclohexanol was subjected to the reaction conditions (in CH3CN), the crude product although colored was found to contain mostly (80%) the 3° acetate.
Acylals (geminal diacetates) have often been used as protecting groups for carbonyl compounds because they are stable to neutral and basic conditions. Acylals can also be converted into other functional groups, adding to their synthetic utility. Herein we report that iron(III) tosylate is also an efficient catalyst for the acylation of a range of aldehydes under mild conditions. The reaction was carried out under solvent-free conditions in most cases. Acetophenone failed to yield any acylal.
Project # 3
Work in progress
Utility of iront(III) tosylate and iron(III) triflate in addition of indoles to chalcones
The conjugate addition of indoles to chalcones can generate a variety of useful substituted indoles with biological activities. We have recently discovered that iron(III) tosylate is a promising catalyst for the conjugate addition of indoles to a variety of chalcones. The full scope of this reaction is now being investigated (Scheme 4).
Scheme 4
Copyright © 2014 American Chemical Society