58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI351964-UNI3: Utilizing Carboxylic Acids to Promote Rapid and Selective Iron Catalyzed Epoxidations of Alkenes
Christopher Goh, PhD, AM, BSc, Williams College
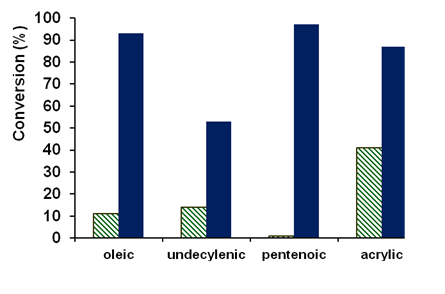
The initial goal of the project was to examine the influence of the presence of a carboxylic acid functional group in alkenes on the rate and selectivity of the epoxidation of these carboxy-alkenes. In this first year of the grant, we focused on using the known epoxidation catalyst [(bpmen)Fe(OTf)2] (bpmen = N,N'-dimethyl-N,N'-bis(2-pyridylmethyl)-1,2-diaminoethane) to oxidize a series of alkenes bearing a carboxylic acid functional group, namely oleic acid, undecylenic acid, 5-hexenoic acid, 4-pentenoic acid and acrylic acid, with hydrogen peroxide as the oxidant. Comparisons with the analogous ester substrates demonstrated the beneficial impact of the acid functional group on conversion and selectivity (Figure 1).
Figure 1: Comparison of the conversion of ester (green, lined) and corresponding acid substrates (blue, solid) demonstrating the positive impact of the acid functional group on product formation.
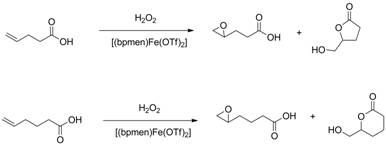
For the oleic, undecylenic and acrylic acids, epoxide product is formed with moderate to high conversions and high selectivities. Under the conditions employed, 4-pentenoic acid is oxidized to a g-lactone, most likely via the epoxide intermediate, and 5-hexenoic acid initially to a mixture of epoxide and d-lactone. The epoxide product then converts to the d-lactone.
Scheme 1: Oxidation of 4-pentenoic and 5-hexenoic acids leading to formation of epoxide and lactone.
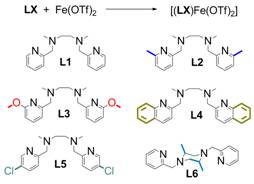
To probe the impact of ligand structure on the catalysis, we synthesized 5 derivatives of the iron-bpmen complex: 4 complexes with varying substitutions on the pyridine rings and 1 with a substituted piperazine backbone.
Figure 2: Iron complexes synthesized to probe the impact of changes of the ligand framework on oxidation catalysis.
Single crystal analyses showed the impact of the substitution on the solid state structures. The iron centers possess distorted octahedral geometries, owing to the steric requirements of the three 5-membered chelate rings of the tetradentate ligands. The two pyridine donor moieties of the tetradentate ligands are trans to each, and the ligands adopt the cis-a conformation typical of iron complexes with bpmen-type ligands. Of the derivative complexes, only the N,N'-dimethyl-N,N'-bis(5-chloropyridin-2-ylmethyl)-1,2-diaminoethane variant showed appreciable activity. It appears that increasing the steric bulk of the ligand in the ortho position of the pyridine rings significantly reduces oxidation activity.
With the start of the second year of the grant period, we continue to probe how changes in conditions and changes in the ligand framework impact the efficiency of oxidation. We are also examining the possibilities of polymerizing the epoxides generated in this study so far.
The impact of this PRF-funded research project has been multifold. It has permitted me to support the research of the following students during the academic year and the summer. Michael Girouard completed a semester-long independent research study (Fall 2012). After graduating this summer, he currently works as a clinical research coordinator at the Medical Practice Evaluation Center (MPEC) at Massachusetts General Hospital. Tamuka Chidanguro (Spring 2013, funded summer 2013) joined the lab as a sophomore student, and plans to continue his efforts as a work-study student. Lilli Morris (funded summer 2013) continues her research in my lab as a senior thesis project this academic year. She presented her results at Williams and at the ACS National Meeting in New Orleans (Spring 2013). With this grant supporting the purchase of supplies, we were also able to provide research opportunities for two additional students, Jeff Brewington and Areli Valencia, who obtained funding for salaries from other sources. Areli, an ACS Scholar, continues his summer research as a second senior thesis student working on this project. Both Areli and Lilli are considering graduate school and careers in teaching undergraduates. 4 of the 5 students involved to date are first-generation students and 3 are from underrepresented minorities. The PRF grant supports our group's continued efforts of synthesizing groups of closely-related ligands which are also used in other homogeneous catalysis projects, thus contributing to the research infrastructure. I recently submitted materials for my application for tenure, and the results from this PRF funded research comprised a significant portion of my scholarship.
Copyright © 2014 American Chemical Society