58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND351921-ND3: Photochemistry and Reactivity of Heterosubstituted Maltol Chelates
Patrick J. Farmer, Baylor University
This PRF funded work concerns a family of new chelating dyes derived from maltol, an FDA approved food additive that has the smell of cotton-candy. Metal complexes of these dyes display unusual reactivity and photochemistry, for example initial studies showed that two dithiomaltol (ttma) complexes, [Ru(bpy)2(ttma)]+ and Zn(ttma)2, act as potent photo-reductants. To date have synthesized several families of metal complexes of hetero-substituted maltol derivatives including Pt(bpy)dye+, Ti(dye)xCly, and certain homoleptic complexes which show photochemical activity. Our goal is to illuminate the nature of aromaticity in these compounds when complexed with metal ions, as well as to correlate aromaticity with the observed photochemical and reactivity characteristics.
Project 1. Ligand-based Photooxidations of [Ru(bpy)2(ttma)]+ and Zn(ttma)2
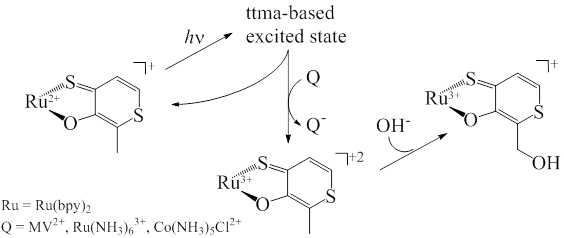
The dithiomaltolato complex [Ru(bpy)2(ttma)]+ has previously been shown to undergo an unusual C-H activation upon outersphere oxidation, generating alcohol and aldehyde products from C-H oxidation of the pendant methyl group.[i] We find that the same products are formed upon photolysis of 1 in presence of mild oxidants such as methyl viologen, [Ru(NH3)6]3+ and [Co(NH3)5Cl]2+, which do not oxidize 1 in the dark, Scheme 1.
Scheme 1
This reactivity is engendered only upon excitation into an absorbance band attributed to the ttma ligand.[ii] Analogous experiments with the homoleptic Zn(ttma)2, 2, also result in reduction of electron acceptors upon excitation of the ttma absorbance band. Complexes 1 and 2 exhibit short-lived singlet and long-lived NIR emissions, as well as long lived transient absorbance changes on the millisecond timescale. Singlet oxygen is both generated and quenched during aerobic excitation of both 1 and 2, but is not involved in the C-H activation process.
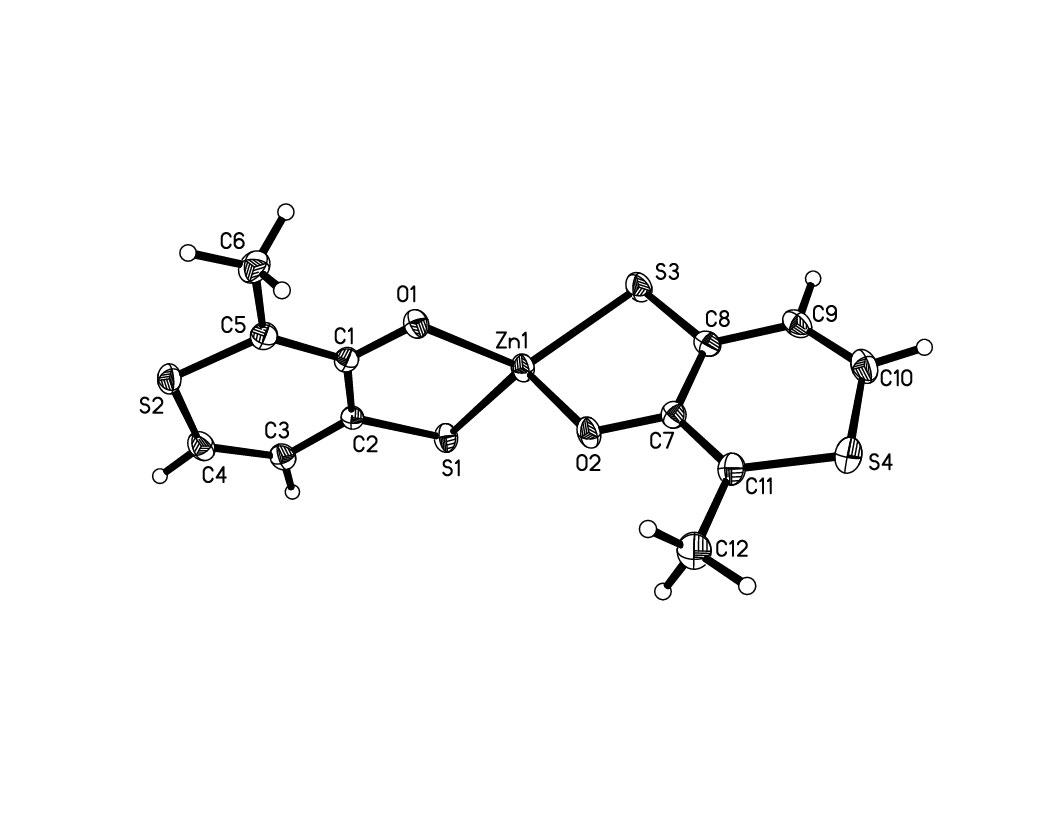 Figure 1. Crystal structure of Zn(ttma)2,
2.
Figure 1. Crystal structure of Zn(ttma)2,
2.
Project 2: Synthesis and photochemical studies of Pt hydroxypyrathionone
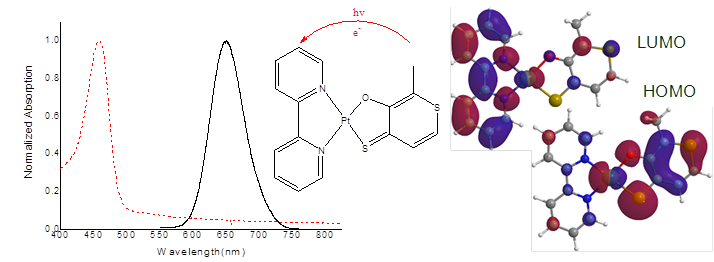
Thiolated hydroxypyrones in [Pt(bpy)L]+, where L = thiomaltolate (tma) or dithiomaltolate (ttma), have an intense ligand based luminescence that facilitate a photo-induced electron transfer to an electron accepter such as methylviologen, Figure 2. To systematically examine the activities of various hydroxy-pyrone ligands in the Pt(II) complexes, a library of ligands were proposed, synthesized and then characterized. [Pt(bpy)(ttma)][PF6] shows high 1O2 sensitivity with a quantum yield near unity. A long-lived emission signal at room temperature is also reported with changes in the excited-state lifetimes and 1O2 sensitivity dependent of ligand variations.
Figure 2. From left, normalized absorption /emission of Pt(bpy)2(ttma)+; schematic of ligand-to-ligand charge transfer; DFT calculated densities of ttma-based HOMO and bpy-based LUMO orbitals.
Calculations and examinations of equilibrium geometries have been performed using the hybrid density functional B3LYP with the def2-TZVP basis set on Turbomole 6.0 package. Full population analysis and NBO calculations were done using the B3LYP method with the basis set def2-TZVP for all atoms and include the def-ECP for the core electrons of Pt. The solvent effects of CH3CN were included using CPCM method; all these calculations were carried out on a Gaussian 03 package.
Project 2. Titanium complexes of heterosubstituted maltols
One possible use for these maltol-based compounds may be in Dye Sensitized Solar Cells, as photoreductants absorbed onto TiO2 particles at the anode surface. Therefore, we examined Ti complexes of the dyes to model their interaction on TiO2. Following a previously reported synthesis of Ti(maltol)2Cl2,[iii] several compounds such as Ti(ttma)Cl3 and Ti(tma)2Cl2 have been isolated from these reactions and crystallographically characterized, Figure 3. The absorbances of these complexes match well to the color of the dyes absorbed on TiO2.
| ttma |
| tma |
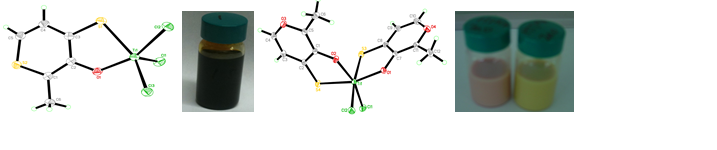
Figure 3. Left, structure of Ti(ttma)Cl3, and dark red solution; center, structure of Ti(tma)2Cl2; right, dyes adsorbed on TiO2 in aqueous solution.
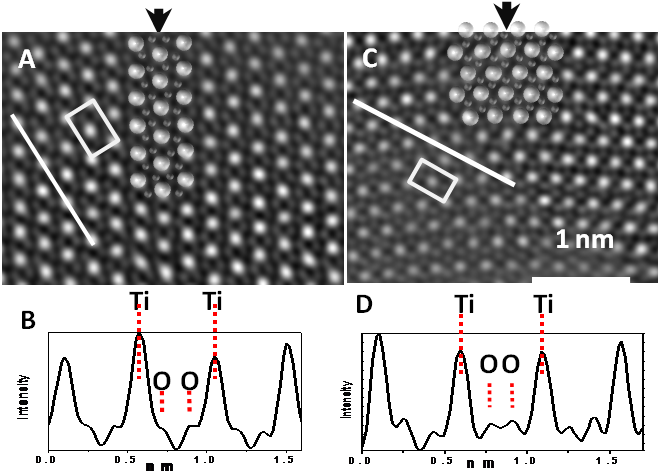
As part of this work, rutile nanotwins were synthesized using high temperature organic solvent methods, yielding two kinds of common high-quality rutile twinned nanocrystals, (101) and (301) twins, accompanied by minor rutile nanorods. In a collaborative effort with the Yacaman lab at UTSA, the atomic structures of the rutile and anatase nanocrystals were directly resolved without need for calculation or image simulation using atomic resolution STEM techniques, Figure 3. The locations of the oxygen rows in the rutile twins' boundaries are directly determined from both HAADF images and ABF images. To the best of our knowledge, this is the first time oxygen columns have been distinguished in rutile twin boundaries using HAADF and BF imaging.[iv]
Figure 3. Processed HAADF images of a rutile (101) and a (301) twins. A) (101) twins, the brighter features correspond to Ti row; the less bright features are O rows, intensity profile aligns the white line. The arrow indicates the position of the (101) twin boundary. B) intensity of profile line shows distances between titanium and oxygen rows in image A. C) (301) twins. The arrow indicates the position of the {301} twin boundary. D) intensity of line profile shows distances between titanium and oxygen rows in image C.IV
[i] Backlund, M.; Ziller, J.; Farmer, P.J. “Unexpected C-H activation of Ru(II)-dithiomaltol complexes upon oxidation" Inorg. Chem. 2008, 47, 2864-2870.
[ii] Bruner, B.; Walker, M.B.; Van der Veer, W.; Zhang, D.; Selke, M.; El-Bjeirami, O.; Omary, M.A.; Klausmeyer, K.; Farmer, P.J. “Ligand-based Photooxidations of [Ru(bpy)2(ttma)]+ and Zn(ttma)2” submitted, under revision.
[iii] Sobota, P.; Przybylak, K.; Utko, J.; Jerzykiewicz, L. B.; Pombeiro, A. J. L.; Guedes da Silva, M. F. C.; Szczegot, K. “Syntheses, Structure, and Reactivity of Chiral Titanium Compounds: Procatalysts for Olefin Polymerization” Chem. Eur. J. 2001, 7, 951-958.
[iv] Lu, W.; Bruner, B.; Casillas, G.; Mejia-Rosales, S.; Farmer, P.J.; Jose-Yacaman, M.; “Direct oxygen imaging in titania nanocrystals” Nanotechnology 2012, 23, 335706.
Copyright © 2014 American Chemical Society