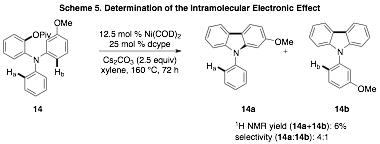58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UNI352224-UNI3: Nickel Catalyzed C-H Arylation using C-O Electrophiles
Dipannita Kalyani, PhD, St. Olaf College
Nickel-Catalyzed C–H Arylation Using C–O Electrophiles
The overall goal of the proposed research is the development of methods for the construction of C–C bonds by nickel-catalyzed coupling of phenolic electrophiles with arene C–H bonds. The use of nickel catalysis for direct arylation will represent a significant advance over the known methods for C–H arylation that use precious metal catalysts. Additionally the use of phenolic electrophiles in place of aryl halides for such transformations is more desirable from an environmental standpoint.
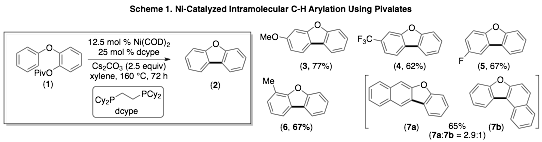
Our studies toward accomplishing the Ni-catalyzed C–H arylation using C–O electrophiles began with the optimization of the intramolecular C–H arylation using pivalate substrate (1). As shown in Scheme 1, the desired transformation could be accomplished using the Ni(COD)2/dcype catalyst system. The reaction is general to afford a variety of electronically differentiated benzofuran products in modest to good yields. Furthermore the optimal reaction conditions could be applied toward the synthesis of carbazoles.
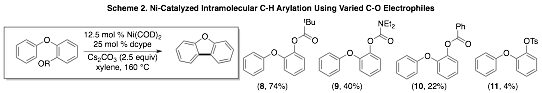
We have also explored the efficiency of these reactions with diverse C–O electrophiles. As shown in Scheme 2, pivalates (8) and carbamates (9) are the most efficient electrophiles under the optimal conditions thus far. Currently we are exploring reaction parameters to improve the efficiency of the transformations with other C–O electrophiles including sulfonates, carbamates and carbonates.
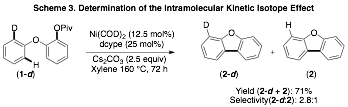
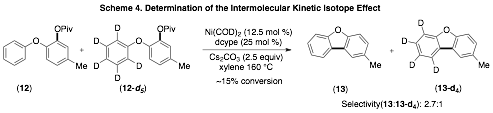
During this year we have conducted a brief exploration for the mechanism of the Ni-catalyzed intramolecular arylations depicted in Scheme 1. Specifically we have conducted the inter- and intramolecular kinetic isotope effect (K.I.E) studies to gain insight into the C–H activation step of these transformations. As illustrated in Schemes 3 and 4, we observed similar K.I.E values for both the inter- and the intramolecular studies. While further studies are needed, the observed kinetic isotope effect is similar to those observed for similar Pd-catalyzed reactions thought to proceed via a concerted deprotonation-metallation C–H activation step.
We have also explored the electronic effect of the intramolecular C–H arylation using substrate 14. Pivalate 14 has two electronically different arene C–H bonds (Ha and Hb) that could undergo functionalization under the reaction conditions. As depicted in Scheme 5 the reaction displays modest selectivity for formation of 14a via the arylation of the more electron rich C–H bond.
The majority of the work described herein has been disseminated in a peer-reviewed manuscript over the past year. Importantly, four undergraduate students have thus far been trained in my research group as part of accomplishing the goals of the proposal. Three of these undergraduate students aspire to pursue a Ph.D. in chemistry while one student desires to obtain M.D./Ph.D. in the chemical sciences. Furthermore, both the PI and the undergraduate students have presented the work described herein in regional and national chemistry conferences.
Copyright © 2014 American Chemical Society