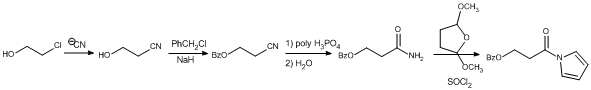58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR452071-UR4: Hydrogen-Bonding Control of Solvatochromism and Non-Radiative Decay in the Fluorescence of PRODAN Derivatives
Christopher J. Abelt, PhD, College of William and Mary
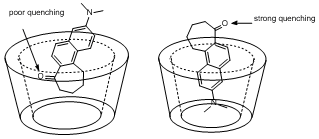
The effects of H-bonding on the fluorescence of PRODAN and derivatives have been the subject of two publications acknowledging PRF support. In the first, the behavior of PRODAN in supramolecular complexes with beta-cyclodextrin is reported. It was shown that the quenching behavior, as reflected in the integrated fluorescence intensities, and the Stokes shift did not change equally in the cyclodextrin complexes. Lack of quenching was attributed to the PRODAN's carbonyl oxygen being buried inside the hydrophobic cavity of cyclodextrin where it could not participate in H-bonding. Lack of a significant Stokes shift was interpreted as the result of continued solvent exposure of the fluorophore. Separating the Stokes shift and the magnitude of the quenching allowed for greater insights into the structure of the complex.
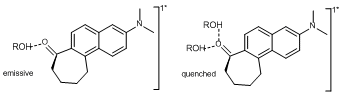
In the other publication (technically, resubmitted with minor changes that did not require further review) characterization of the preferential solvation of alcohols was used to help determine the structure of the species responsible for the strong quenching behavior. The preferential solvation model of Roses and Bosch was augmented to allow for characterization of the singly H-bonded fluorophore in terms of its Stokes shift and relative quantum yield. It was discovered that a single H-bond led to ~three-fourths of the ultimate Stokes shift but almost no change in the relative quantum yield compared to the non H-bonded fluorophore. The strong quenching in these systems requires two H-bonds. This result has significant implications for the direction of this grant.
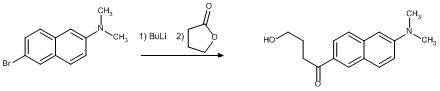
Synthesis of model compounds incorporating one H-bond have begun. Two synthetic schemes have been tried and abandoned. In the first, the scheme failed at the nitrile hydrolysis. The ultimate product would have undergone an elimination reaction in the presence of an aryl lithium instead of the desired substitution at the carbonyl.
The second was more troubling because it is based on relatively straightforward methods. The synthesis worked up to the last step involving the deprotection of the alcohol. Hydrogenolysis of benzyl ethers should be simple, but even with Pd catalyst designed for such reactions, the benzyl group remained intact. Tweeking the reaction with acid did result in deprotection, but it reduced the carbonyl as well.
A different protecting group will be tried this year.
Finally, a relatively simple model compound having an intramolecular H-bond was prepared using gamma-butyrolactone.
Solvatochromism studies are planned for this year. It is anticipated that these systems may form cyclic hemi-ketals under irradiation. Such reactions would result in loss of fluorescence intensity with continuous irradiation.
Copyright © 2014 American Chemical Society