58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR550594-UR5: Fundamental Studies of Diffusion and Retention on Porous Polymer Monolith Stationary Phases Used in Capillary Electrochromatography
Michelle Marie Bushey, Trinity University
Organic porous polymer monoliths (PPM) are a new type of stationary phase that differ significantly in structure and performance from traditional particulate phases and even from silica based monoliths. We are investigating the behavior and performance of these new stationary phase materials with capillary electrochromatographic and HPLC approaches to better understand the PPM at a fundamental level. By better understanding these materials, we will through comparison, also better understand the behavior of traditional phases. These types of studies have not been undertaken on organic PPMs before. Our studies are focused on lauryl acrylate crosslinked with 1,3-butanedioldiacrylate.
Our investigations have focused on two areas, the relationship between diffusion and retention as defined by a rearrangement of the Knox equation (1) and through thermodynamic studies of retention. The Knox equation relates the diffusion of the analyte in the presence of the stationary phase (Deff), to the diffusion of the analyte in the mobile phase (Dm), the obstruction factor of the column (gm), the kinetic parameter (gsDs), and the retention factor (k). To measure the diffusion of analytes in columns with or without a stationary phase, the analyte is moved to the center of the column and the driving flow, in this case voltage, is paused for different lengths of time. The variance of the peak obtained in experiments without the pause is subtracted from the variance obtained in experiments with a 'parked' time. The difference is the variance due to the axial diffusion of the peak.
(1 + k) Deff /Dm = gm + kgsDs/Dm (1) s2 = 2Dt (2)
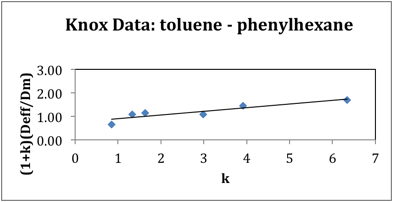
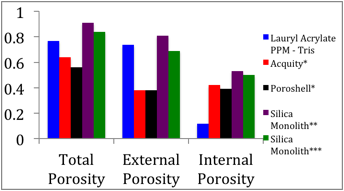
We have modified our methods for measuring diffusion of analytes in columns by including a brief stop in all runs to be able to account for the variance due to the starting and stopping of the driving field. Doing so has improved our data set as evidenced by a better adherence to linearity. All previous investigations of stationary phases comprised of C18 bonded to silica, in monolithic or particulate columns, have reported nonlinear Knox plots. Our improved Knox plot is shown below in Figure 1. The intercept of this plot yields the obstruction factor of the column. This value can also be determined through the ratio of Deff/Dm for a non-retained species. We previously reported a value of 0.72 (+/- 0.01) for nonretained thiourea (Anderson et.al. Electrophoresis (2010) 31, 1583). The intercept obtained in Figure 1 for retained species is 0.74 which is in good agreement with our previous report. Porosity measurements have been completed and those experiments clearly indicate that this PPM material has greatly diminished internal porosity relative to the external porosity. This is in contrast to other types of stationary phases reported on in the literature. These differences are highlighted in Figure 2. We maintain that it is this greatly diminished internal porosity, resulting from a lack of mesopores, which explains the linear Knox plot behavior.
Thermodynamic data is shown in Table 1. This is obtained by measuring the retention of analytes across a temperature range. The relationship between the retention factor (k) and enthalpy (DHo) and entropy (DSo) of partitioning is given by the van't Hoff equation (3). The enthalpy is obtained from the slope and the entropy from the intercept, provided the phase ratio (F) is known. Our system allows for reliable phase ratio measurement (on a nanoflow HPLC) and it has been determined to be 0.189 +/- 0.002. The enthalpy and entropy values given in the table show that retention is enthalpically driven and that there is a relatively large increase in order associated with the transfer of analyte from the mobile phase to the stationary phase.
ln k = DHo/RT – DSo/R + F (3)
To date, ten undergraduates have worked on this project. Four of these students have been directly supported by this funding. Five of the ten have been able to participate not only during the academic year but also during two summer research seasons. One former student is now in graduate school seeking a PhD in biochemistry at Boston University. A second is employed as a lab technician here at Trinity University. Seven have yet to graduate and five of them continue to work on this project during the current academic year. The last is seeking employment overseas.
Students supported by this grant in the summers of 2011, 2012, or 2013 gave presentations at Pittcon in Philadelphia, Mar. 17 - 21, 2013. Their travel was partially support by these funds. Other students and one postdoctoral associate working on this project but supported through other funds gave an additional three presentations at the same conference. The postdoc and students have four presentations accepted for presentation at Pittcon 2014.
An invited talk was presented at the Latin American Capillary Electrophoresis Symposium in Buenos Aires, Argentina, Nov. 30 – Dec. 3, 2012 and a second will be presented at this conference in Lima, Peru, Nov. 30 – Dec. 3, 2013.
Figure 1. Knox plot for alkyl benzene series. Diffusion-retention relationship.
Figure 2. Porosity comparisons.
*Liekkens, Denayer, Desmet. J Chrom A (2011) 1218, 4406.
**Miyabe, K.; Matsumoto, Y.; Guiochon, G. Anal. Chem. (2007) 79, 1970.
***Miyabe, K. J. Sep. Sci (2006) 29, 2452.
Table 1. Thermodynamics of retention on lauryl acrylate PPM.
| ΔH
| ΔS
| ΔG 25°
| ΔG 60°
|
(kJ/mol) | (J/moláK) | (kJ/mol) | (kJ/mol) | |
Toluene
| -4.61
| -3.48
| -3.58
| -3.45
|
Ethyl Benzene
| -5.27
| -3.44
| -4.24
| -4.12
|
Propyl Benzene
| -6.37
| -4.33
| -5.08
| -4.93
|
Butyl Benzene
| -7.24
| -4.49
| -5.91
| -5.75
|
Amyl Benzene
| -8.65
| -6.28
| -6.77
| -6.55
|
Hexyl Benzene
| -9.85
| -7.40
| -7.64
| -7.38
|
Heptyl Benzene
| -11.07
| -8.55
| -8.53
| -8.23
|
Octyl Benzene
| -12.29
| -9.67
| -9.41
| -9.07
|
Copyright © 2014 American Chemical Society