58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: ND150983-ND1: Exploring Indolidene Chemistry: New Opportunities for Complex Indole Synthesis
Ken Feldman, Pennsylvania State University
The ACS/ND grant funded the continuing efforts to develop indolidene chemistry as a strategic entry point into the synthesis of complex alkaloids. Two different areas of research were pursued: (1) the elaboration of the scope of the formal [4+3] cycloaddition to give cycloheptanone-annelated indoles, and (2) the continued pursuit of the alkaloid target gilbertine via indolidene chemistry.
The formal [4+3] cycloaddition of an indolidenium intermediate with an electron rich diene was first discovered during the previous year of ACS/PRF funding. We have recorded three subsequent successes to begin to establish the scope of this transformation, Fig. 1. The putative indolidenium intermediate 2, readily available from alcohol 1 in the presence of a mild Lewis acid, combines effectively with Danishefsky's diene 3 and the less electron rich and more sterically encumbered dienes 4a/4b to give the cycloheptenanone-containing products 5 and 6 in excellent yields following aqueous acid workup. These complex product structures highlight the potential value of this emerging methodology for the total synthesis of cognate alkaloids like ambiguine G and actinophyllic acid.
Figure 1. New formal 4+3] cycloaddition chemistry of indolidenium intermediates.
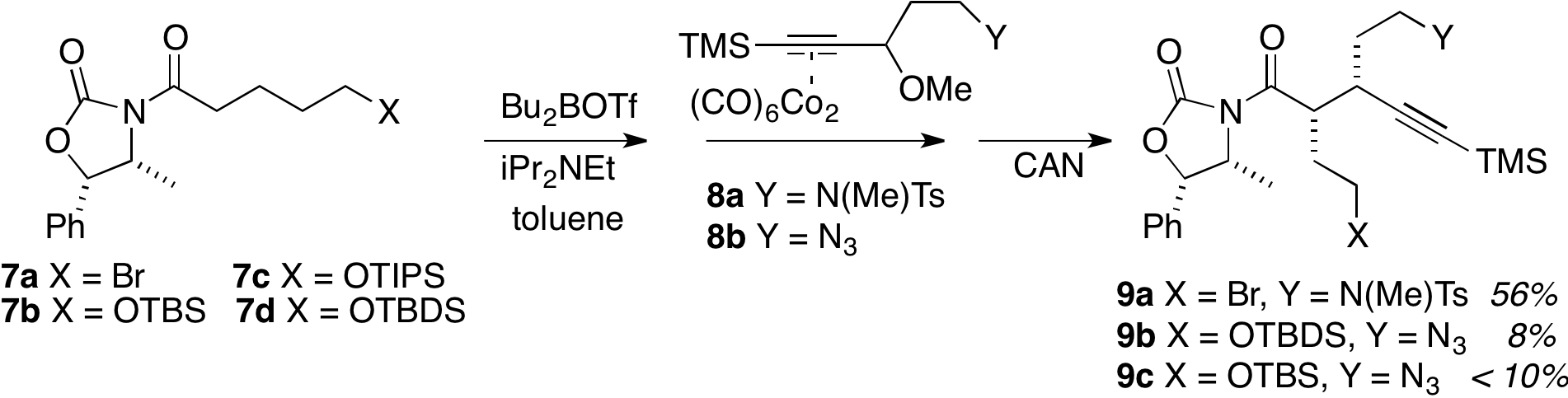
The application of indolidene chemistry to the total synthesis of gilbertine is still in the "key substrate" synthesis stage. We have devoted much effort toward yield optimization of a key transform in the synthesis route, Fig. 2. Exploring several variations of the pivotal Nicholas reaction between the cobalt complexes 8a or 8b and the functionalized enolates derived from 7a – 7d ultimately led to the conclusion that the original (from the previous Progress Report) pairing of X= Br and Y = N(Me)Ts was the most effective.
Figure 2. Optimization of the Nicholas procedure.
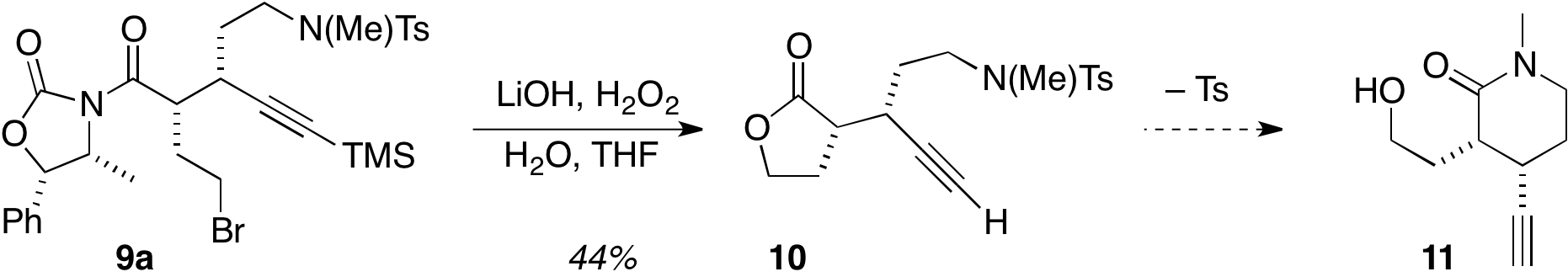
Continuation of the synthesis effort using the bromide/N(Me)Ts product 9a has afforded the desilylated lactone 10. Efforts are currently underway to deprotect the amine, which should promote cyclization to give the desired lactam 11, Fig. 3. The final target gilbertine should be available from 11 in 5 more steps.
This work has served as a training vehicle for a graduate student and two undergraduate coworkers. These students are gaining experience in the art and practice of organic synthesis, reaction optimization, spectroscopic analysis, and synthesis route planning. The discoveries described above, especially in Fig. 1, open up a new research thrust in my group that at present looks very promising. Many aspects of the formal [4+3] cycloaddition have yet to be explored, and I anticipate that this problem will be catalytic in problems.
Copyright © 2014 American Chemical Society