58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI252128-DNI2: Understanding Factors that Control Gas Hydrate Stability: Does Microbial Activity Induce Gas Hydrate Dissolution?
Laura Lapham, University of Maryland Center for Environmental Science
Progress-
This project tests the hypothesis that aerobic methane oxidizing bacteria (methanotroph) enhance gas hydrate dissolution by synthesizing methane hydrate in the laboratory and exposing it to a pure culture of aerobic methanotrophs. Experiments carried out include 1) determining what conditions are optimal for a pure culture of methanotroph to grow, 2) growing the culture under high pressure and low temperatures, suitable for methane hydrate formation and 3) growing them under high pressure while slowly decreasing temperature to optimize growth. We have determined that the culture grows under high pressure. In year 2, the goal is to carry out the main experiment of this project which is to determine gas hydrate dissolution rates in the presence of this methanotroph.
Introduction
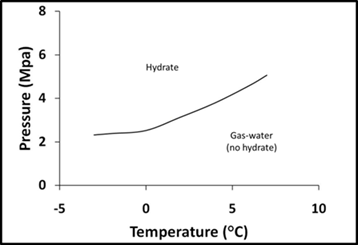
Gas hydrates are crystalline ice-like solids that contain methane gas, CH4, and form naturally in Arctic permafrost and marine deep-sea sediments. They are the largest reservoir of CH4 on Earth and the main component of natural gas (1). As such, the resource potential for hydrates is great (2) and the need to understand factors controlling their stability even greater. Hydrates are stable when high pressure, low temperature, and saturated CH4 conditions exist (Figure 1). When these pressure and temperature conditions are not met, they will dissociate, releasing CH4 gas to the surrounding environment. But when these pressure and temperature conditions are met, hydrate stability is controlled by CH4 concentrations immediately surrounding them. If these concentrations fall below saturation, the hydrate will dissolve via molecular diffusion to re-establish saturated conditions (3). This dissolving CH4 could then be utilized by methanotrophic bacteria which oxidize CH4 aerobically:
CH4·6H2O (s) = CH4 (aq) = CO2 (1)
where CH4·6H20(s) is pure methane hydrate and CH4 and H2O(aq) are dissolved CH4 and water, respectively. CO2 is carbon dioxide formed by aerobic methane oxidation. Methanotrophs could dissolve hydrate by pulling equation 1 further to the right. This process will be addressed by synthesizing gas hydrate in a pressure vessel, introducing a pure culture of methanotrophs, and measuring hydrate dissolution rates.
Pressure vessel
A Parr© pressure vessel was purchased in late Fall 2012 (Figure 2). Vessel details are presented in Lapham et al. (4). Briefly, the 600mL stainless steel vessel holds ~20MPa pressure. There are two viewing ports on the side, pressure and temperature sensors on the top, and sampling ports on the bottom. Gas hydrate was successfully synthesized by adding a pure methane headspace to 300mL of microbial media (data not shown).
Analytical techniques
CH4 concentrations were measured on a gas chromatograph equipped with a flame ionization detector and Poropak Q and Mol Sieve packed columns (5). Stable carbon isotope ratios were measured on an isotope-ratio-mass-spectrometer equipped with a GC (5). Microbial growth was quantified by measuring cell densities in water samples on a spectrophotometer set at ~405nm wavelength (cell densities are proportional to absorbance).
Microbial culture
In January 2012, a pure culture of a methane oxidizing bacteria, Methylmicrobium album, was obtained and reconstituted with simple salts media at its optimal growing conditions, 30°C and 1atm. The culture was placed into sterile glass vials with media and a 50%:50% mix of methane in air and sealed. Absorbance was then monitored to determine exponential growth took ~7 days. The culture was then expanded and stored in 1mL aliquots of 50% glycerol at -80°C for future experiments.
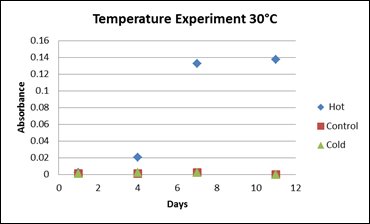
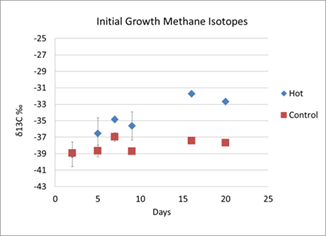
Since the culture will ultimately grow at gas hydrate conditions (Figure 1), the culture was then grown at several different temperatures and pressures. First, at 30°C and 1atm pressure, microbial growth was observed in the “hot” treatment after 6 days (as indicated by an increase in the absorbance, Figure 3). Methane oxidation was verified through the use of stable carbon isotopes (Figure 4). As methane is oxidized, the residual methane becomes more enriched in the heavier isotope, 13C, leaving the ratio (d13C-CH4) more positive in value than the control (Figure 4). Thus, stable isotopes are a sensitive measure of the amount of methane being oxidized (6). At lower temperatures, the culture did not grow (data not shown).
Cultures were then grown in the pressure chamber at 3.4MPa and 6°C, conditions just outside of the hydrate formation range. Lasting 6 weeks, this experiment showed no growth in the chamber (data not shown). To determine if the culture was still viable, a sub-sample of pressure vessel media was collected and allowed to grow at 1atm and 30°C in a glass vial (data not shown). Surprisingly, the culture grew under these conditions, suggesting that the culture was still viable under these high pressures and low temperatures.
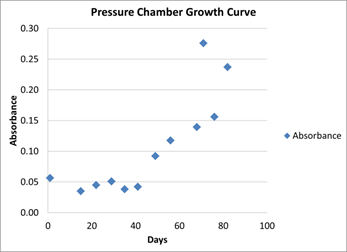
With these promising results, the culture was grown at high pressure and high temperature. Therefore, at 3.4MPa and 30°C, the culture reached exponential phase within 40 days (Figure 5). This suggests that temperature, not pressure, is the limiting factor to cell growth.
Future
For year 2, the main experiment will be carried out. Methanotrophs will be grown in the presence of gas hydrate to determine how they affect gas hydrate dissolution rates. Using start-up funds to supplement the PRF funds, a second pressure chamber will be used in year 2. References
1. Sloan, 1998. Clathrate hydrates of natural gases, 2nd edition.
2. Boswell et al., 2012. Marine and Petroleum Geology 34 4-30.
3. Zhang and Xu, 2003. Earth and Planetary Science Letters 213 133-148.
4. Lapham et al., 2012. Rapid Communications in Mass Spectrometry 26 32-36.
5. Lapham et al., 2013. Geochemistry, Geophysics, Geosystems.
6. Liptay et al., 1998. Journal of Geophysical Research 103 (D7), 8243-8250.
Figure 1: Pressure and temperature plot for hydrate
Figure 2: Pressure vessel
Figure 3: Microbial growth curves at 30°C. “Hot” treatments (blue) are inoculated media with 50% methane: 50% air headspace. “Control” treatments (red) are inoculated media with only air headspace. And “cold” treatments (green) are media not inoculated with bacteria but contain 50% methane: 50% air headspace
Figure 4: Stable carbon isotopes of methane in "hot" and "control" treatments at 30°C.
Figure 5: Growth of bacteria under high pressure (3.4 MPa), high temperature (30°C) conditions.
Copyright © 2014 American Chemical Society