58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR349503-UR3: In Situ Thermodynamic Characterization of Supramolecular Assembly Processes that Lead to Discrete Nanosize Structures
Douglas A. Vander Griend, Calvin College
In our quest to study chemical systems with high degrees of organizational, dynamic, and compositional complexity, we have several new major scientific results to report this year. For details of previous results, please see the annual report submitted in September of 2012.
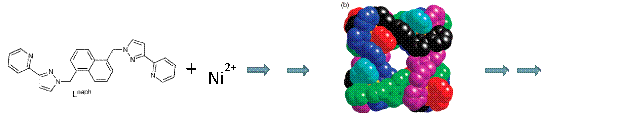
We continue to make progress using spectrophotometric titrations to investigate the self-assembly of the twenty molecular pieces that form into a supramolecular cube (Figure 1). The original chemistry was designed by Dr. Michael D. Ward of Sheffield University. This represents the most complicated self-assembly process characterization that we or anyone else has undertaken as far as we know.
Figure 1. Twelve Lnaph ligands (two bidentate pyrazolyl–pyridine termini separated by a 1,5–naphthalene) and 8 nickel(II) cations form a cubic nanocage in acetonitrile or DMF solution.
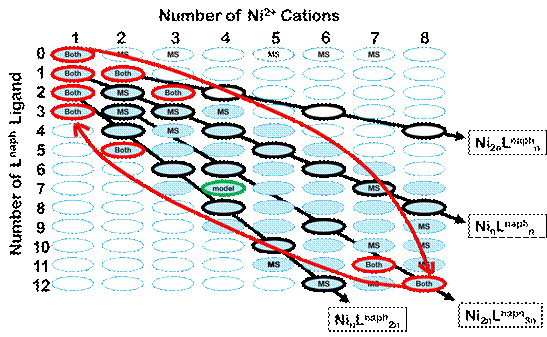
Modeling spectrophotometric titration data with our computer program Sivvu evidenced 8 distinct sub-assemblages other than Ni(II) and the complete cube assembly. High resolution mass spec data, taken at Michigan State University, corroborated all but one of these sub-assemblages (Figure 2). Together the results from UV-vis titrations and mass spec show that most sub-assemblages are quite small or nearly a completed cube. This points to the fidelity of the cube which is well-designed to be considerably more thermodynamically stable than any other possible arrangements of the ligand and metal in solution.
Figure 2. The titration of Ni(BF4)2 with Lnaph leads to many potential species: NinLm n = 1-8 and m = 0-12. Shaded ovals correspond to the set of assemblages that can hold together with coordinative bonds in a single unit: up to nickel cations per ligand and up to 3 ligands per nickel. Assemblages that were evidenced by mass spectrometry (MS), modeling of spectrophotometric titration data (Model), or both are outlined in bold. Straight black arrows connect sets of assemblages that share a common Ni:Lnaph ratio and are therefore difficult to differentiate. Red curved arrows indicate the order of appearance of assemblages during the course of a titration of nickel cation with ligand.
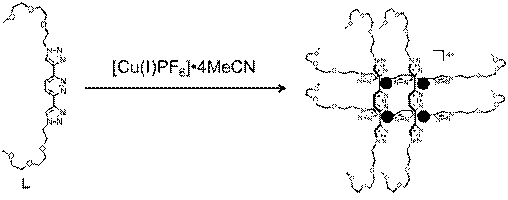
A second major project dealing with supramolecular chemistry has come to completion with impressive results. Prof. Flood and I have submitted a manuscript that details the step-by-step assembly in solution of an 8 piece grid molecule, shown in Figure 3.
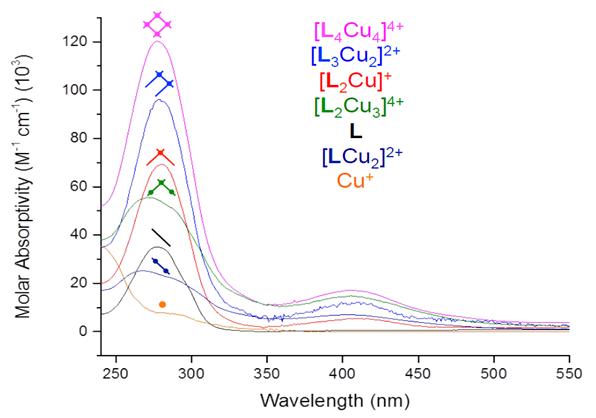
Figure 3. Four equivalents of bis-bidentate ligand (LMeetP = 3,6-bis(1-(2-(2-(2-methoxyethoxy)ethoxy)ethyl)-1H-1,2,3-triazol-4-yl)pyridazine) coordinate to 4 equivalents of Cu(I) in to form a 2 × 2 grid supramolecular complex.
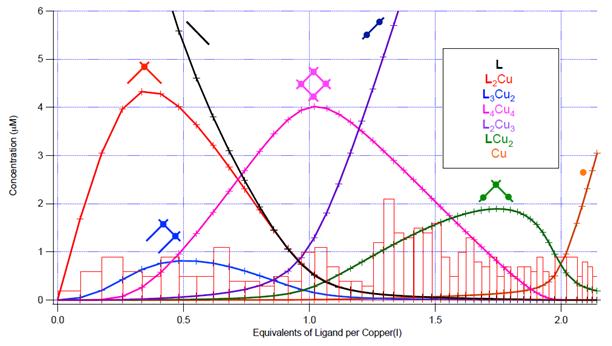
Equilibrium-restricted factor analysis was applied to a set of 50 UV-Vis spectra generated by titrating a 20 μM solution of LMeetP in dichloromethane with 2.14 equivalents of copper(I). Concentration profiles (Figure 4) and molar absorptivity curves (Figure 5) for the seven absorbers evidenced in this system together account for 99.21% of the raw absorbance data (0.62% of the data is random noise). The extinction coefficient spectra of each species correlate well to the number of ligand absorbers (ε280) and the number of metal-to-ligand charge transfer transitions (ε400). The concentration profiles represent the change in free energy for the step-by-step reactions involved in assembly and disassembly. These values are accurate enough to establish that the coulombic price for bringing the four monocations together in a grid in this system is no higher than 30 kJ/mol.
Figure 4. Concentration profiles for the 50 solutions with LMeetP and copper(I). Bars represent the root-mean-square residual for each solution in the ERFA model.
Figure 5. Molar absorptivity values for the seven absorbing species present in the series of solutions. L is LMeetP.
A complete set of complementary 1H NMR scans are consistent with the rich set of complexes and equilibria involved in the assembly and disassembly pathways. Altogether this study represents one of the most consistent characterizations, spectroscopically and thermodynamically, that has even been achieved for a system as complex as this.
Also this year, we concluded our investigation of the solution dynamics of the dye methylene blue with a publication in Analytical Chemistry, and I also finally published got some older data taken by a former undergraduate student researcher on the stepwise coordination of nickel(II) with pyridine and methylpyridines. In addition, we began studying several new systems this summer. 1) protein folding, 2) Iron(III)/thiocyanate system, and 3) copper(I)/phenanthroline.
With 1 returning student, 2 new ones and a high school student, the lab was very busy this past summer. All five of us visited Indiana University, Bloomington in order to meet with the graduate group of Dr. Amar Flood, who is one of my collaborators. The shared presentations and interaction with graduate students proved quite formative for the Calvin undergrads as they all began to positively envision themselves in chemistry graduate school. All four students continue to conduct research now that the school year has begun and are quite eager to bring their respective projects to a conclusion.
I am pleased to report, that even as my ACS-PRF grant is now completed, the research will continue as I have won a grant from NSF for three more years of funding.
Thank you for supporting this research and making the impact possible.
Copyright © 2014 American Chemical Society