58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: UR552049-UR5: Synthesis and Multilayering of Poly(4-vinylphenol) Derivatives for Future Applications as Proton Exchange Membranes in Fuel Cells
Ronny Priefer, PhD, Western New England University
Firstly, the important hypothesis of the grant is that having matching pKa’s of the two multilayered polymers is the key factor that has produced our strong conductivity results. However, it has been well established that once polymers are incorporated into multilayer systems their pKa’s will change to either a higher or lower value. This was determined by multilayering polymers with varying layer numbers onto silica colloids and determining their zeta potentials. Since we have pioneered the work on multilayering pseudo-polyelectrolytes our particular systems have never been evaluated. Thus, using 70 nm Snowtex silica particles obtained from Nissan Chemical Industries we successfully multilayered poly(allylamine hydrochloride) with poly(4-vinylphenol). We subsequently have just completed the data collection using our Beckman Coulter’s Delsa Nano C Particle Analyzer. Although, at the time of this report the data analysis is not complete, the PI can confidently state that the pKa’s of poly(allylamine hydrochloride) and poly(4-vinylphenol) are indeed very close when multilayered at six total layers and higher. This validates our initial hypothesis. This will be written up for submission as a publication within the year.
Secondly, we have been able to synthesize some of the polymers as delineated within the grant, however a derivative of poly(4-vinylphenol) that we had always believe inaccessible was the 2-acetyl compound. This was initially believed to be not possible since our synthetic route utilizes a Wittig reaction. We however have been able to show moderate success with a photo-Fries rearrangement of poly(4-acetoxystyrene). We still need to optimize this reaction, however we are very encouraged with the results to date.
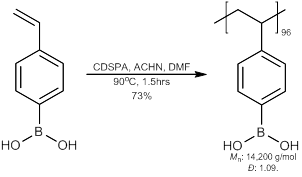
Thirdly, in the first few months in the move while the PI was setting up the lab and chemistry could not begin immediately, thoughts of employing other pseudo-polyelectrolytes was considered. Again, the matching pKa’s was a crucial consideration. Thus poly(4-vinylbenzeneboronic acid) was envisioned. Although not commercially available, its synthesis has been previously reported. We slightly modified the procedure by incorporating reversible addition-fragmentation chain transfer (RAFT) polymerization to allow for a narrower polydispersity. Therefore, 4-vinylbenzeneboronic acid, 4-cyano-4-[(dodecylsulfanyl-thiocarbonyl)sulfanyl] pentanoic acid (CDSPA), 1,1’azobis(cyclohexanecarbonitrile) (ACHN), in a 1.0 : 0.045 : 0.0016 ratio was dissolved in N,N-dimethylformamide (DMF), subjected to three freeze-evacuate-thaw cycles, sealed under vacuum and heated to 90oC for 1.5 hours (Scheme 1). The mixture was diluted with methanol, precipitated twice with ether, and vacuum dried at 40oC overnight, to afford the polymer with a Mn of 14,200 g/mol with a polydispersity of 1.09.
Scheme 1: RAFT polymerization for the synthesis of poly(4-vinylbenzeneboronic acid) (PVBBA).
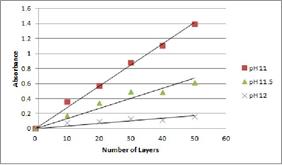
We have also been able to start to evaluate the potential of poly(4-vinylbenzeneboronic acid) as a pseudo-polyelectrolyte in multilayer films. As with poly(4-vinylphenol), it was required to initially dissolve in an elevated solution pH then lower to the desired acidity. Due to the low UV-Vis absorbance profile of the multilayered system, it was necessary to create the films on quartz slides. The poly(allylamine hydrochloride)/poly(4-vinylbenzeneboronic acid) have an absorption spectrum with two distinct peaks at 200 and 230 nm (Figure 1) with a linear growth at all the assembly pHs evaluated (Figure 2). The surface morphology (Figure 3) is similar to that of the poly(allylamine hydrochloride)/ poly(4-vinylphenol) system. However, the thickness profile data are still being compiled with the PI’s collaborator, Dr. Todd Krauss from the Department of Chemistry at the University of Rochester. Once the thickness has been determined we will analyze the conductivity of these systems (thickness measurements are necessary for determining conductivity) with the PI’s collaborator, Dr. Matthew Yates from the Department of Chemical Engineering at the University of Rochester. It is envisioned that this work will soon be ready for publication.
Figure 1: Absorption profiles measured for PAH/PVBBA multilayered system deposited at pH 12.0 with 10, 20, 30, 40 and 50 layers.
Figure 2: Absorbance (at 230 nm) of PAH/PVBBA multilayer film systems measured after addition of new film layers created at a pH of 11.0, 11.5, 12.0.
Figure 3: AFM images showing film topography of PAH/PVBBA multilayered system created at pH 11 (a) 10, (b) 20, (c) 30, (d) 40, (e) 50 layers. The z-limits range from 200 to 300 nm.
Copyright © 2014 American Chemical Society