58th Annual Report on Research 2013 Under Sponsorship of the ACS Petroleum Research Fund
Reports: DNI151247-DNI1: The Development of Enantioselective Friedel-Crafts Reactions to Prepare Unnatural Amino Acid Derivatives
Sarah Reisman, PhD, California Institute of Technology
Tryptophan and unnatural tryptophan derivatives are important building blocks for the total synthesis of natural products, as well as the development of new drugs, biological probes, and chiral small molecule catalysts. As part of our research program aimed at establishing new methods for the enantioselective synthesis of alkaloids, we became interested in developing convergent syntheses of tryptophans and cyclo-tryptophans (also known as pyrroloindolines) from simple indole starting materials. Our research funded by this Doctoral New Investigator award from the ACS PRF has resulted in the first direct, enantioselective synthesis of tryptophan derivatives by a tandem Friedel–Crafts conjugate addition/asymmetric protonation reaction.[1] These reactions require no pre-activation of the indole substrates, and provide convergent access to a range of substituted tryptophan derivatives in enantioenriched form.
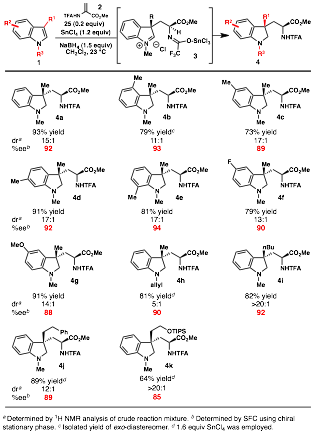
Since completing our studies on the enantioselective synthesis of tryptophan derivatives, we have been engaged in two follow-up areas of research. Mechanistic studies of the reaction between 1,3-dimethylindole and benzyl 2-trifluoroacetamidoacrylate revealed the presence of a persistent iminium ion (3) under the reaction conditions.[2] To further expand the types of products accessible through these Friedel–Crafts conjugate addition/asymmetric protonation reactions, we sought to trap this iminium ion with 1) hydride sources, and 2) carbon-based nucleophiles. Towards the first objective, a variety of hydride sources were studied. Whereas weaker reductants such as triethylsilane and sodium triacetoxyborohydride proved ineffective, use of sodium borohydride furnished 4a in good yield, 15:1 dr, and 92% ee. The more soluble reducing agent lithium borohydride provided a lower yield of the desired product along with a greater amount of byproducts. The limited solubility of NaBH4 and LiBH4 in methylene chloride likely contributes to the compatibility of all the reagents, allowing the reaction to be carried out in one pot.
Having identified an optimal reducing agent, a survey of indole substrates was conducted (Table 1). Indoles with either electron-donating or -withdrawing substituents are good substrates for the reaction. At the 3-position, n-butyl, phenylethyl, and TIPS-protected alcohol groups are tolerated, but reactivity decreases with increasing steric bulk, and 1.6 equivalents of SnCl4 are required to achieve good yields. The indole nitrogen can also be be protected with an allyl group, making this method more useful when an N-Me functionality is not desired in the product.
Table 1. In situ reduction for the synthesis of indolines.
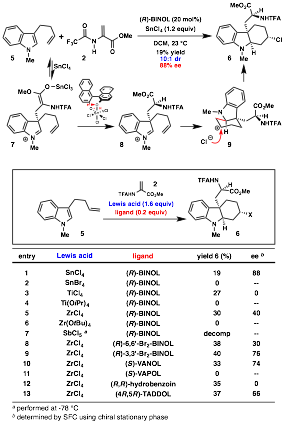
In the second line of research, trapping of the transiently formed iminium ion with carbon nucleophiles has been investigated. Thus, we found that exposure of homoallyl indole 5 to standard reaction conditions yielded indoline 6, the product of an aza-Prins cyclization with chloride trapping of the carbocation, albeit in low yield (Scheme 1). A screen of Lewis acids showed that ZrCl4 was also effective for this reaction (Scheme 1, entry 5). While the ee was lower, we opted to continue optimization with ZrCl4 as it provided a qualitatively cleaner reaction profile. A variety of chiral diol ligands were also screened, and (R)-3,3'-Br2-BINOL was found to give the highest ee (entry 9).
Scheme 1. Conjugate addition/Prins cyclization: Initial discovery and reaction optimization.
In our tryptophan synthesis, the proton at C3 of the indole is proposed to turn over the deprotonated BINOL catalyst;1 however, in the present system, the correlation between yields and catalyst loading suggested that catalyst turnover was problematic. Based on precedent from Yamamoto's studies on the catalytic asymmetric protonation of silyl enol ethers by an (R)-BINOLŸSnCl4 complex,[3] we hypothesized that addition of an external stoichiometric proton source would facilitate turnover. Careful optimization of the achiral proton source would be required to minimize the rate of unselective enolate protonation. A variety of substituted phenols were screened: wheras 2,6-dimethylphenol improved the yield, the ee was lower (Table 2, entry 3) due to competitive unselective enolate protonation by the phenol. On the other hand, the more hindered 2,6-tBu2-phenol failed to improve yield (entry 4), indicating that proton transfer from the phenol to the BINOLate was too slow.
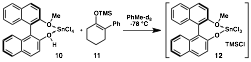
Scheme 2. Yamamoto's NMR studies.
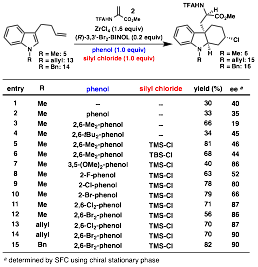
At this point we returned to mechanistic studies performed by Yamamoto on his protonation of silyl enol ethers.3 NMR studies of the reaction promoted by a stoichiometric amount of the Lewis acid-assisted Brznsted acid revealed formation of a tin-aryloxide species 12 and TMSCl (Scheme 2). In the catalytic reaction, the tin complex is proposed to receive a proton and chloride from 2,6-dimethylphenol and TMSCl, respectively, and TMS-2,6-dimethylphenol is formed as a byproduct. Based on these results, TMSCl was added to our conjugate addition/Prins cyclization, which indeed resulted in catalyst turnover (Table 2, entry 5). After further screening of the reaction parameters, N-benzyl substrate 14 was found to be optimal; use of 2,6-Br2-phenol provided Prins product 16 in 82% yield and 90% ee. Our future research in this area will seek to determine the substrate scope of this transformation and to further develop tandem Friedel–Crafts conjugate addition/enantioselective protonation/cascade cyclization reactions. This research has established that a variety of value-added unnatural amino acid derivatives can be derived from indole, a simple petroleum-based starting material.
Table 2. Screening of reaction parameters.
Importantly, this award has provided students with the opportunity to conduct exciting research in the area of asymmetric catalysis. Several members of my group have worked on various aspects of this project, and it has proven to be a fertile area for both mechanistic studies and synthetic applications. The support provided by this DNI award has positively impacted my career by providing me with the opportunity to pursue exciting, fundamental research in the area of asymmetric catalysis. I expect that the findings described here will continue to drive an exciting area of research in my laboratory for many years to come.
Copyright © 2014 American Chemical Society