AmericanChemicalSociety.com
Reports: GB5 47286-GB5: Density Functional Theory and Car-Parrinello Molecular Dynamics Simulations of Ethanol Electro-oxidation Reaction on Bi- and Trimetallic Nanoparticles
Yixuan Wang, Albany State University
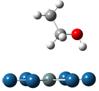
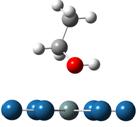
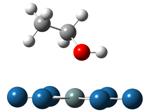
Adsorption and decompositions of ethanol over PtnM clusters (n=6 and 9; M=Pt, Sn and Ru) have been systematically investigated to get the insights into the ethanol electro-oxidation reaction (EER) mechanisms. Although trans-ethanol is more stable than the cis- one, as a result of the adsorptions in all of the investigated bimetallic PtM the latter turns out to be a more favorable adsorption as previously reported for Pt. The adsorption via the hydroxyl is much stronger than that via the α-hydrogen. As shown below for ethanol adsorption over cluster Pt6Sn, the adsorption energies are -1.40, -1.36 and -0.74eV for the two hydroxyl adsorptions and α-hydrogen, respectively.
(Eads: -1.40eV) (Eads: -1.36eV) (Eads: -0.74eV)
Figure 1. Three major adsorptions of ethanol on Pt6Sn:
via hydroxyls of cis-ethanol (left) and trans-ethanol (middle), and
α-hydrogen (right)
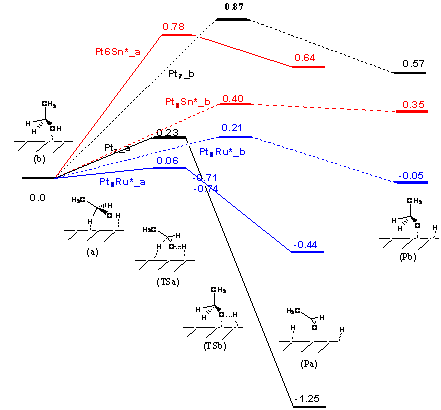
The comparison of the adsorptions on the three clusters Pt6M
(M=Pt, Sn, and Ru) indicates that the adsorptions of both hydroxyl and
α-hydrogen on the central Sn and Ru of Pt6M are much stronger
than those on the central Pt of Pt7. The decomposition behaviors for
the two hydroxyl adsorptions are rather similar to each other, for an example,
the energy barriers to the adsorbed CH3CH2O* and H* on Pt7
are 0.87 and 0.89 eV, respectively. It is interesting to note that on the
clusters Pt7 and Pt6Ru the decomposition resulting from the
α-hydrogen adsorption has lower energy barrier than those of the hydroxyl
adsorptions (0.23 eV vs 0.87/0.89eV for Pt7; 0.06 eV vs 0.21/ 0.36eV),
while on the cluster Pt6Sn oppositely the α-hydrogen
decomposition has higher barrier than the hydroxyl dissociation (1.05 vs
0.57/0.59eV). The results indicate whether ethanol decomposition proceeds
initially via O-H or α-C-H bond cleavage may depend on the type of catalysts.
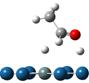
(adsorption via α-hydrogen
) (decomposition TS) (decomposition product)
Figure
2. Dissociative adsorptions for hydroxyl and α-H of ethanol over Pt-based
bimetallic: A concerted pathway
The adsorption via
α-hydrogen considerably extends C-H bond by 0.1~0.2Å, and hydroxyl also
has significant adsorption with the Pt-H(O) distance of approximately 2.4 Å.
Therefore, the transition state in Figure 2 may be characterized as the TS for
the co-decomposition of hydroxyl and α-hydrogen, which decompose in a
concerted pathway. According to Figure 3, the favorable decomposition pathway (
via α-hydrogen) on Pt7 has a lower activation energy by 0.17eV
than the favorable pathway (via hydroxyl) on Pt7Sn, and
thermodynamically the former is also favorable than the latter ( dissociation
energy: 0.35 vs -1.25eV). Thus, Pt is more active to ethanol oxidation reaction
than Sn. Together with the investigation of H2O adsorption on PtnM,
the present results support the proposed bifunctional mechanism for PtSn alloy
to catalyze ethanol electro-oxidation reaction that the dissociative adsorption
of ethanol occurs on platinum sites and Sn primarily promotes adsorption and
dissociation of water to form OH oxidizing intermediates from ethanol.
Figure 3. Potential energy profile for the
decompositions of ethanol on Pt6M (M=Pt, Sn and Ru)

Copyright © American Chemical Society