AmericanChemicalSociety.com
Reports: ND7 49158-ND7: A Graft Semiconductor Approach to Novel Materials for Photovoltaic Applications
Elsa Reichmanis, PhD, Georgia Institute of Technology
Technique Approach
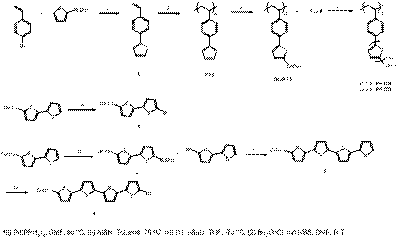
As an alternative to silicon based cells, π-conjugated organic materials have been shown to be attractive candidates in electronic devices because of their compatibility with high through-put, low cost processing techniques, and their capability to be precisely functionalized through the techniques of organic synthesis to afford desired performance attributes. Most π-conjugated linear polymers possess rigid aromatic backbones that serve as the charge transport medium. Potential issues surrounding the use of such conjugated polymers arise such as twisting when those materials are used in PV cells comprised of ordered rigid electron acceptors. An alternative approach to overcome these problems is to synthesize polymers with semiconducting units grafted onto a flexible backbone allowing for good solubility and necessary π-stacking among the grafted side chains.
Herein we try to synthesize oligothiophenes grafted polystyrenes with the pendant conjugation systems perpendicular to the polymer backbone. To avoid steric hindrance that prevents a direct free radical polymerization of the oligothiophene-modified styrene monomer to form high molecular weight graft polystyrene, 2-(4-vinylphenyl)thiophene (5) was first synthesized and polymerized to form poly(thiophenyl styrene) (PTS). The PTS precursor can be easily modified to produce a stannylated derivative (Sn-PTS). The oligothiophene semiconducting units can be then grafted onto the polystyrene backbone to form the graft polymers PH3TS and PH5TS via Stille coupling reaction as shown in Scheme 1.
Scheme 1. Synthetic route of PH3TS and PH5TS.a
Preliminary Results Molecular
weights of the resulting polymers were determined by gel permeation
chromatography (GPC) as listed in Table 1.
The dry PTS is a light yellow
powder (Mn = 13000, PDI = 2.01) and very soluble in common organic
solvents such as DCM and THF. The
resulting polymer PH3TS obtained
from the grafting reaction is a yellow powder (Mn = 19700 and DPI =
2.16), which is soluble in many common organic solvents such as diethyl ether,
THF, DCM, and chloroform. The polymer PH5TS is a reddish powder, partially
dissolved in THF, CHCl3 or tri-chlorobenzene to form a reddish
suspension. Table 1. Summary of properties of the polymers PTS, PH3TS and PH5TS.
Polymers Mn Mw PDI Td (oC) TDSC (oC) PTS 13000 26300 2.02 325 155 (Tg) PH3TS 19700 42600 2.16 325 n/a PH5TS 28100 63400 2.25 298 n/a The thermal
curves of PH3TS and PTS seem very similar, with the same decomposition
temperature (5 wt % loss) at 325 oC by TGA. Compared to them, PH5TS demonstrates lower thermal stability with Td at
298 oC. There was no clear
evidence of any usual glass and crystallization behavior for both polymers as
detected by DSC and hence the polymers were assumed to involve highly hindered
segmental motions and appeared to be amorphous.
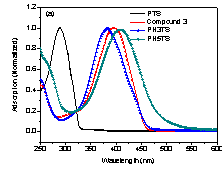
The normalized
UV-Vis absorption spectra of PTS, PH3TS and PH5TS in chloroform are reported in Figure 1. As it was expected, PH5TS with the longest conjugated side chain in this study exhibits
a more red-shifted peak absorption maximum of 407 nm while PH3TS and PTS exhibit
the absorption maximum at 383 and 293 nm, respectively. Figure 1. UV-Vis
absorption spectra of Polymers PTS, PH3TS and PH5TS in CHCl3 (~0.1 mg/mL). To study the
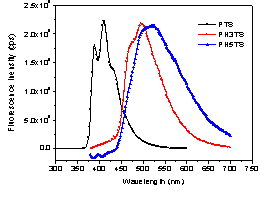
intra- and inter- molecular interactions, the polymer solution was studied by
fluorescence spectroscopy. As shown in
Figure 2, these polymers show fluorescence emissions in the visible region upon
excitation at a certain wavelength (275 nm for the PTS, 375 nm for the PH3TS
and PH5TS). A progressive red-shifted wavelength was
found as the chain length of the grafted oligothiophenes increased. Emission peaks were observed at 411, 485 and
514 nm for the polymers PTS, PH3TS, and PH5TS, respectively. But an
apparent difference in the shape of the emission peak of PTS was observed compared to those of PH3TS and PH5TS. The peak of PTS was split into two sub-peaks at 387 and 409 nm with a shoulder
at 431 nm, indicating strong inter-chain interactions. Figure 2. Fluorescence spectra of polymers in CHCl3. As shown in
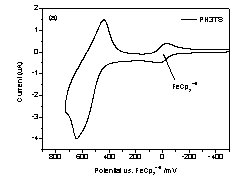
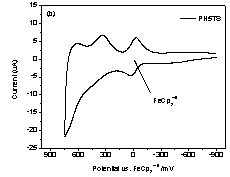
Figure 3, only oxidation peaks were revealed for both polymers by cyclic
voltammetry. The onset of the oxidation
occurred at approximately 0.46 V (vs. FeCp2+/0) and
reached maximum at 0.64 V (vs. FeCp2+/0). As for PH5TS,
due to its limited solubility in DCM, a uniform film could hardly be deposited
onto the platinum electrode. It resulted
in an irreversible oxidation process with decreased anodic current in
subsequent scan cycles. The HOMO energy levels were calculated using the
equation
Figure 3. Cyclic
Voltammograms for a) PH3TS and b) Ph5TS. Table 2. Optical and electrochemical properties of
polymers.
λabs(nm) Egopt (eV) E1/2+/0 EHOMO ELUMO PH3TS 383 2.60 0.54 -5.34 -2.74 PH5TS 424 2.37 0.42 -5.22 -2.85 Summary
and Future Plan We have shown a
rational molecular strategy to design and synthesize semiconductor grafted
polymers by grafting π-conjugated oligothiophenes onto a polystyrene
backbone. The thermal stability, optical
and electrochemical properties have been studied. However, due to its limited
solubility of PH5TS in common
organic solvents, it is very difficult to fabricate organic thin-film
transistor devices out of this polymer in a solution process for a consistent performance.
The solubility of this material needs to be enhanced in the future. This can be realized by attaching longer
alkyl side chain onto the pendant thiophene groups. Meanwhile, a spacer will be inserted between polymer
backbone and each grafted semiconducting core.
It will provide a way to study how flexibility of pendant semiconducting
cores impacts packing among the grafted side chains and future facilitate
charge carrier mobilizing. Additionally, including the oligothiophene, new
semiconducting structure will be explored in the future. For examples,
diphenylene dithiophene is a well studied organic semiconductor with comparable
mobility and relatively low HOMO level. It will be an attractive building block
for synthesizing new class of air stable semiconducting polymers.
![]()
Copyright © American Chemical Society