AmericanChemicalSociety.com
Reports: AC10 47508-AC10: Strain Engineering of Organic Semiconducting Molecules: A First Principles Study
Feng Liu, University of Utah
Using mechanically induced strain to control the structure of semiconducting organic molecules offers a route to manipulation of their optoelectronic properties. As mechanisms crucial to material function occur at the molecular level, a thorough characterization of the impact of mechanically induced strain on isolated molecules gives valuable insight into the physical processes underlying observed properties. We have taken fluorene oligomers which are known to undergo a structural change at the single molecule level from a randomly twisted glassy phase to a planar ordered β-phase when strained. Density functional theory calculations and Car-Parrinello molecular dynamics simulations have been implemented to systematically study the impact of tensile strain on the vertical transition energies of fluorene oligomers. We have also investigated the implementation of strain through specific adsorption on a silicon (100) substrate, following the same principle as strain engineering for inorganic semiconductors.
The impact of tensile and compressive strain on isolated planar fluorene oligomers was initially investigated. We determined that tensile stretching does not change the molecular shape and the strain is accommodated principally in the C-C bonds linking conjugated fluorene units, the vertical transition energy being blue shifted by 0.12 eV from that for the unperturbed geometry as expected due to a decrease in conjugation over the molecule. Compression, however, results in two possible deformations: bending within and out of the molecular plane respectively, both of which have the same energetic cost. We found that bending within the molecular plane resulted in a red shift by 0.02 eV of the vertical transition energy as the C-C linking bonds are compressed, but bending out of plane resulted in a blue shift of 0.07 eV as the conjugation is decreased. Therefore the vertical transition energies can be shifted in opposite directions for the same percentage compressive strain.
As the planar β-phase is thought to form from the glassy phase when strained, we simulated the stretching of twisted isolated fluorene oligomers. We found that the elongated planar molecule was never more stable than the twisted configuration, and never observed a transition between the two molecular phases. After consideration of factors such as temperature and stretching rate, number of monomers in the oligomer, and side group substituents on the relative stability of the two configurations, we determined that on stretching the molecule becomes linear and adopts an ordered twisted structure in which all dihedral angles correspond to the energetic minimum of 140°. On excitation this structure readily planarizes to the β-phase. However, without the pre-stretching, the molecule will not adopt the β-phase on excitation.
In order to induce strain we investigated the
adsorption of a fluorene dimer and polymer on the Si(100) substrate. The
Si(100) substrate consists of parallel rows of Si dimers and it is known that
unsaturated organics can adsorb, forming covalent bonds with Si dimers via
[2+2] or [4+2] cycloaddition reactions. The mismatch between the length of the
fluorene units and the spacing between Si dimers potentially enables stretching
or compressing the molecules on adsorption. We determined that both a fluorene
dimer and a model polymer adsorbed intact in a planar configuration lying face
down with the molecular axis running along the Si dimer rows. In the case of
the fluorene dimer, the molecule was compressed with the strain accommodated in
a shortening of the C-C linking bone, and for the polymer the molecule was
elongated although the strain was not evenly distributed 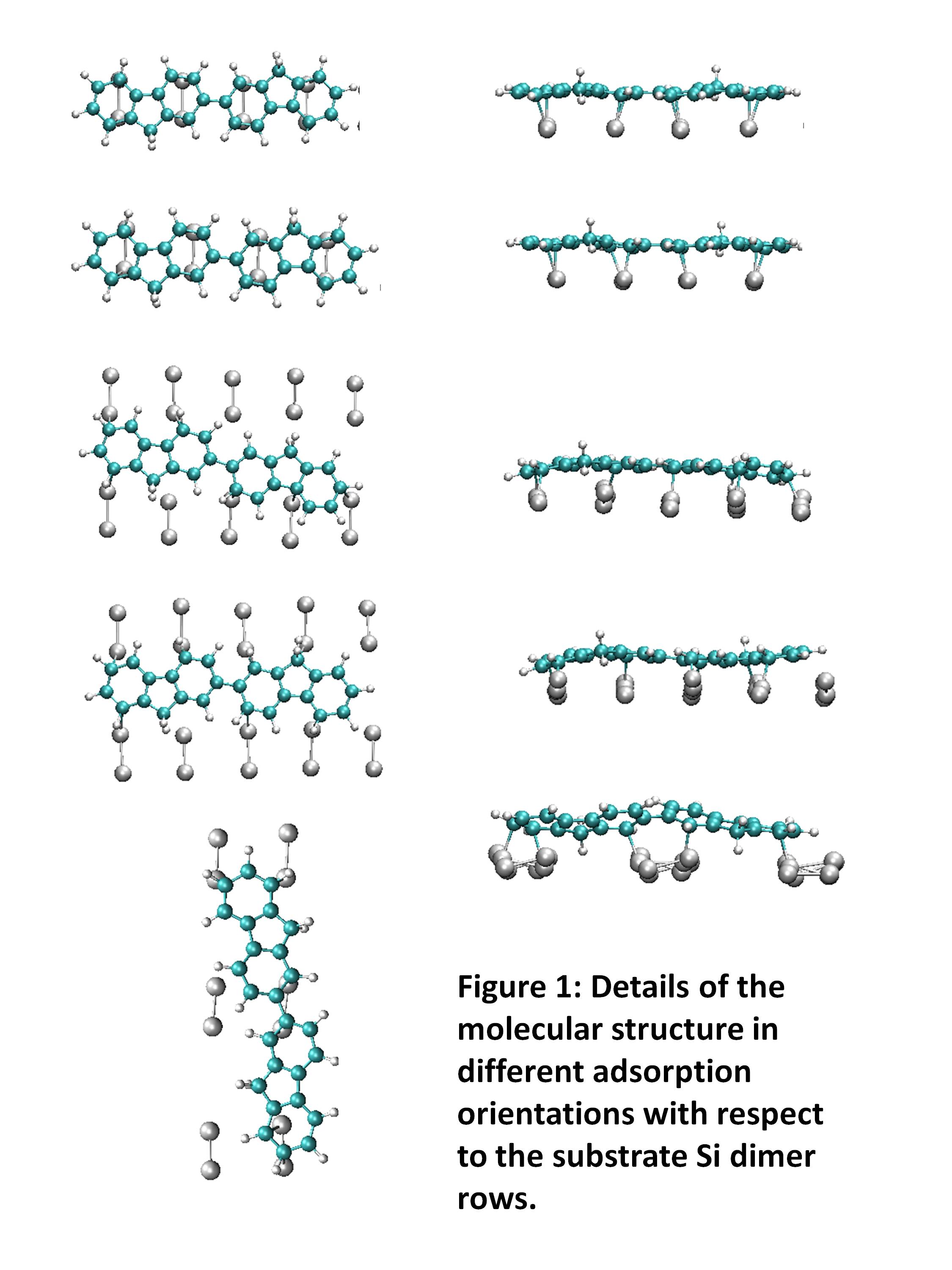 throughout
the molecule.
throughout
the molecule.
In order to determine how different sites and orientations affected the adsorption configuration of the fluorene dimer, we performed a series of density functional theory calculations with the molecule shifted parallel and perpendicular to the Si dimer rows and lying orthogonal to the dimer rows, as well as including a twisted molecular conformation for the starting geometry (fig. 1). For all orientations the molecule adsorbs intact and shifting the molecule parallel to the Si dimer rows was found not to affect the adsorption geometry. In the case of the molecule shifted perpendicular to the Si dimer rows we determined that the molecule bridges two neighboring rows resulting in a slightly different configuration of the molecule with an undulation along the molecular axis. Interestingly we found that starting the molecule in a twisted conformation did not have an impact on the conformation of the adsorbed molecule, which remained planar. However, for the configuration with the molecular axis orthogonal to the Si dimer rows, the molecule was twisted with a dihedral angle of 140° on adsorption. The molecular conformation therefore is more dependent on the orientation of the molecule than the starting conformation.
In conclusion we have completed an extensive study of the impact of mechanically induced strain on isolated molecules to determine how strain is accommodated in structural changes which affect the vertical transition energies. A counterintuitive result is that the shape of the deformed molecule is more important for strain induced changes in the excitation energies than a simple consideration of absolute strain. It is improbable that the planar β-phase of polyfluorenes can be induced by stretching in the ground state of isolated molecules, but is rather formed on excitation of an extended molecule. The planar structure is therefore less important for observation of the pure blue color of the β-phase than the extension of the molecule in the ground state before excitation.
We have also investigated a means for applying strain through specific adsorption where we determined that fluorenes could be strained along their molecular axis. We also found that the molecular distortion was dependent on molecular orientation on the surface. These findings are significant for considering the optoelectronic properties of organic thin films grown on substrates where specific adsorption occurs in the first monolayer. We have published one paper and three further publications are in preparation based on the results of this project.
Copyright © American Chemical Society

