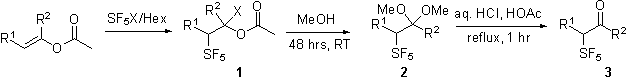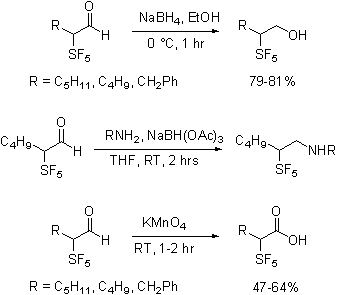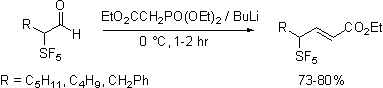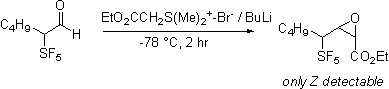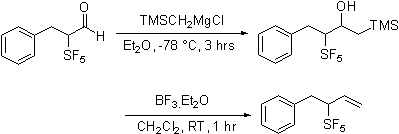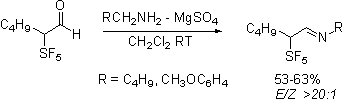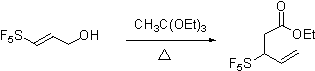Back to Table of Contents
46219-AC4
The Preparation of Optically Active Pentafluorosulfanylated Building Blocks: Selectivity and Reactivity in Synthesis
John T. Welch, State University of New York at Albany
I. Introduction. The
pentafluorosulfanyl (SF5) group is one of only a very few truly new
functional groups to be introduced to the armentarium
of the synthetic organic chemist in the last 100 years. The pseudooctahedral
symmetry of the SF5 group, presenting a square pyramid of electron
density, as defined by the fluorine ligands, is not otherwise known to the
medicinal or pharmaceutical chemist. Although
this functional group has very recently found applications as an aromatic
substituent in agrochemicals, pharmaceuticals and liquid crystals, in aliphatic
chemistry, pentafluorosulfanylated materials are only very rarely encountered
with applications largely limited to polymer or oligomer preparations. The profoundly electron withdrawing nature of
the SF5 group when combined with the highly polarizable
carbon-sulfur bond may directly influence reactivity in manners different from
those associated with the trifluoromethyl group. The hypotheses to be tested in the proposed
research are; firstly that the electronic
influence of the pentafluorosulfanyl unit can be used to direct the
stereochemistry of reactions of a pentafluorosulfanylated molecule and
secondly that pentafluorosulfanylated
building blocks can be converted to carbohydrates, amino acids or other biologically
active molecules.II. . Preparation and Purification of
Starting Aldehydes and Ketones. Over the last year we have focused our
energies on the preparation of the necessary building blocks for the study of
the electronic influence of the SF5 group. The goal was to reproducibly prepare
α-pentafluorosulfanyl aldehydes and ketones. We have learned that the pentafluorosulfanyl bromide
addition product, the acetals and the carbonyl compounds are stable and readily
purified.Table 1.
Synopsis of Preparations.
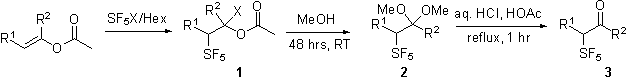
Entry | R1 | R2 | SF5X | Yield
haloacetate 1 | Yield
acetal 2 | Yield
carbonyl 3 | a | C5H11 | H | SF5Br | 43% | 71% | 82% | b | C3H7 | H | SF5Br | 74% | 62% | 24% | c | C4H9 | H | SF5Br | 88% | 76% | 81% | d | PhCH2 | H | SF5Br | - | 76% | X | e | PhCH2CH2 | H | SF5Br | 27% | 91% | 49% | f | H | H | SF5Br | - | 97% | - | g | H | Ph | SF5Cl | - | - | 44% | h | H | C6H13 | SF5Cl | 66% | - | - | i | C2H5 | C3H7 | SF5Cl | 15% | - | - | j | C2H5 | C3H7 | SF5Br | 50% | - | - | k | H | CH3 | SF5Cl | 92% | - | - |
III.
Summary of Findings. Reactions of Aldehydes. The
SF5-containing aldehydes can be subjected to a variety of transformations. To establish the scope and generality of
reactions of SF5-containing aldehydes and the stability of the
products, those aldehydes have been subjected to a variety of routine
transformations, such as sodium borohydride
reduction, reductive amination, and permanganate oxidation.
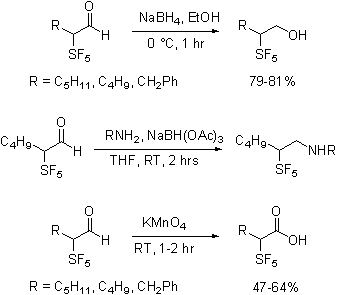
As shown the reactions were
uneventful, in contrast to published findings we have found that Grignard and organolithium additions
to the aldehyde are especially clean reactions.
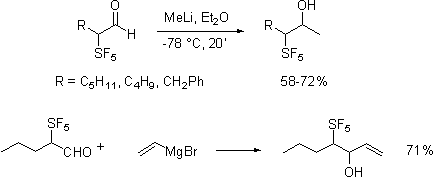
In contrast, olefination
reactions are sensitive to the nature of the reagent employed. Horner-Emmons reagents add to give easily purified
and characterized materials. In contrast
Wittig reagents, phosphonate ylids,
diphenylsulfonium cyclopropylide
and trimethylsulfoxonium ylides
do not react cleanly.
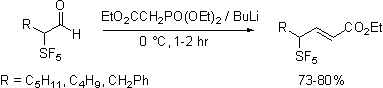
Dimethylsulfonium carboethoxymethylide
does react selectivity forming only the Z expoxide. With the observation that the apparently less
basic ylides react selectively it was found that
Peterson olefination via the intermediacy of silyl
alcohol also proceeds cleanly. Apparently the increased acidity of the proton
alpha to the SF5 group is responsible for the complexity of the
reaction products.
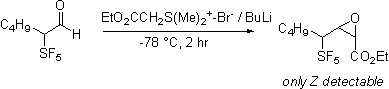
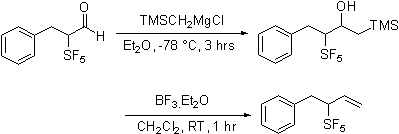
The most exciting evidence of
stereoselectivity influenced by the pentafluorosulfanyl group is in the
Staudinger reaction of the aldehydes. Not
unexpectedly, the required imines are formed stereoselectively.
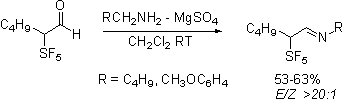
Interestingly the ketene-imine
cycloaddition reaction, occurs selectively forming
only a single diastereomeric pair from the racemic imines.
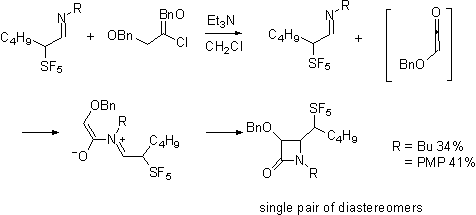
Our current efforts are directed
toward determining whether this product is the lk,ul
diastereomeric pair that would be consistent with the Felkin-Anh
model and with the work of Yamazaki on trifluoromethylated imines.
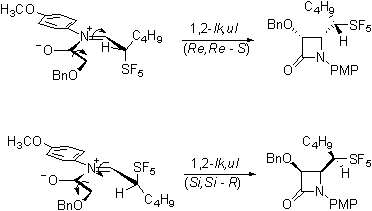
Or is rather the lk,lk
pair that would be consistent with a Cieplak
description of the reaction.
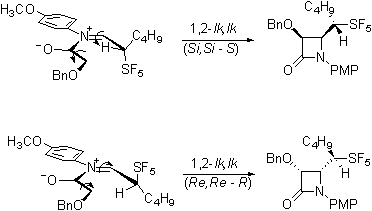
It is important to note that the
exploration of the influence of the pentafluorosulfanyl group on reactivity and
selectivity is slowed as we move further from the initial SF5Br
reagent. It is the is a necessity to
prepare larger and larger amounts of the pentafluorosulfanyl containing
building blocks in order to explore the reaction conditions and stability of
the products that has led us to focus on those reactions that form products
most cleanly to facilitate isolation and characterization. However our work on [3,3]-sigmatropic rearrangements is currently in
progress. We have focused our
preliminary experiments on the Johnson ortho-ester
Claisen rearrangement of pentafluorosulfanyl containing allyl alcohol prepared
as shown.
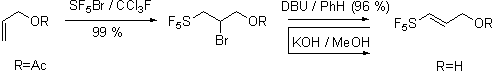
Our preliminary investigations of the Johnson orthoester
Claisen procedure are consistent with the Bronsted acid stability of
aliphatic pentafluorosulfanyl group and indicate that the reaction
proceeds albeit slowly.
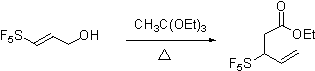
Back to top