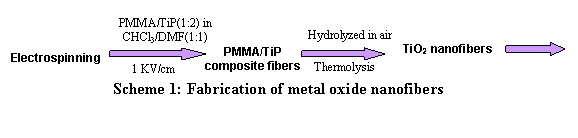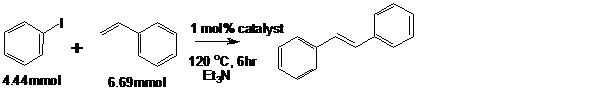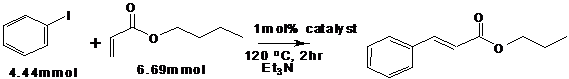Back to Table of Contents
46117-AC5
Nanofiber Catalyst Supports and Solution-Based Processes for Deposition of Catalytic Metals and Metal Oxides
Wayne E. Jones, State University of New York at Binghamton
The adsorption of stabilized
metal nanoparticles on metal oxide nanostructured materials confers profound
prospects to catalysis in organic synthesis.1,2 These include; ease
of recovery and recyclability of these catalysts from reaction medium, large
surface area to volume ratio, as well as stability at high temperatures that
are fundamental in production and refining of petrochemicals.3,4 The
bottom-up approach and solution-based fabrication processes provide low cost
catalysts with a low catalyst loading, good selectivity, and enhanced
reactivity under mild conditions. This is attributed to novel properties
accrued to nanomaterials relative to bulk materials due to quantum level
interactions.5 In phase one of the PRF progress, we report a simple
template based approach via electrospinning of
fabrication of titanium dioxide (TiO2) nanostructures followed by solution
based impregnation and reduction of palladium nanoparticles onto TiO2
nanostructured supports. These catalysts (Pd-TiO2) were applied in
Heck C-C coupling reactions to determine the conversion rate, selectivity and
stability of the catalysts.
Electrospinning provides an
inexpensive, straightforward route to the fabrication of high surface area
fiber membranes.6 This process entails the application of an
electric field to a polymer solution or
composite leading to generation of fibers on nanometer scale, and was exploited
in fabricating nanofibers and nanotubes. Nanotubes were synthesized by a fiber-based
template process, where electrospun fibers
served as a scaffold upon which a metal oxide
precursor was deposited via sol gel
process whereas nanofibers were fabricated via electrospinning a composite
mixture of titanium isopropoxide (TiP)/polymethylmethacrylate (PMMA) as illustrated by the
following schemes.7,8
PMMA/TiP composite fibers |
PMMA/TiP(1:2) in CHCl 3/DMF(1:1) |
aa Scheme 1: Fabrication of metal oxide nanofibers
|
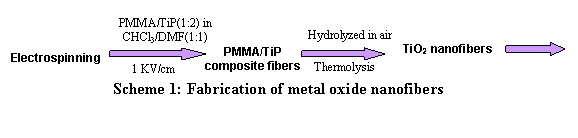




(a)Scanning Electron Microscopy (SEM)
image of electrospun composite PMMA/TiP (b) TiO2
nanofibers after pyrolysis at 400°C, with diameters 150±50 nm
(d) Transmission Electron Microscopy image TiO2 nanofibers (150±50
nm) (d) Pd nanoparticles on TiO2
nanofibers with diameters range 6-10 nm.
The morphology of fabricated composite
fibers was smooth with dimensions ranging between 150±50 nm in
diameter. Pyrolysis temperature, 400°C, was based
upon thermogravimetric analysis of the degradation
profile of PMMA. FTIR and Powder X-ray diffraction confirmed complete removal
of organics leaving behind an anatase crystalline phase of titania. Pd nanoparticles were uniformly distributed with 5% loading and diameters ranging between 6-10
nanometers over the TiO2 support. Nanoparticle impregnation |
Scheme 2: Fabrication of metal oxide nanotubes by templating |
Catalyst supported on nanotube |
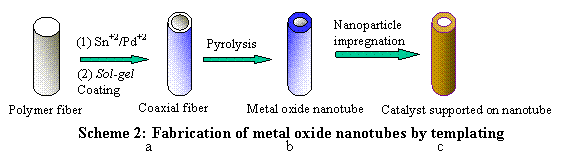



SEM images (a) Electrospun polylactide, PLA, template with diameters 200±50 nm (b) PLA-TiO2
coaxial fibers after sol gel coating
with diameters 250±100
nm (c) TiO2 after pyrolysis at 400 °C,
diameters 200±50 nm and wall thickness
~100 nm.
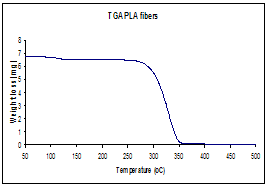
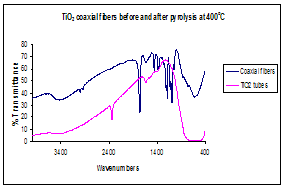
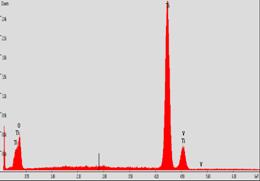
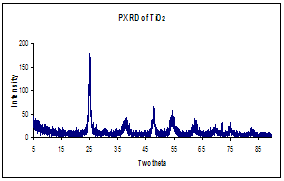
(a)Thermogravimetric analysis,
TGA, illustrating the thermal decomposition of polylactide polymer (b) Infrared
spectrum before and after calcinations indicating
complete degradation of PLA
polymer (c) EDS indicating TiO2 formation (d) Powder X-ray
diffraction indicating anatase crystalline phase of TiO2 nanotube.
Pd-TiO2 catalysts fabricated were tested for Heck C-C coupling of Iodobenzene with
scheme (3) styrene and
scheme (4) n-butylacrylate.9,10
The reactions were carried out in an air and comparisons were made with (a) Pd/C in an inert atmosphere,
(b) Pd(OAc)2 as
unsupported powders.
Scheme 3: C-C coupling of iodobenzene with styrene
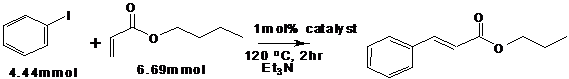
Scheme 4: C-C coupling of iodobenzene with n-butylacrylate
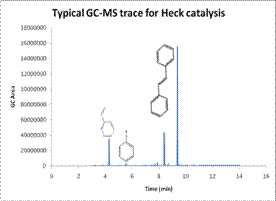
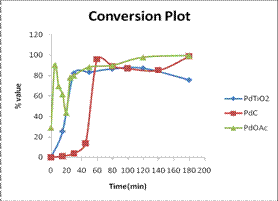
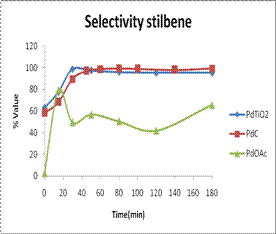
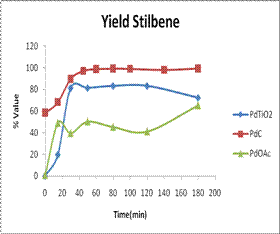
From the GC, Pd-TiO2 showed
higher conversion of reactants to products compared to Pd(OAc)2
complex, and comparable rates to those given by Pd/C (the
commercially obtained catalyst) even though reaction with Pd-TiO2
was manipulated under air atmosphere. Pd-TiO2 catalyst exhibited high activity and selectivity for the
desired product even at 0 minute reaction time, with yields comparable to Pd/C.
The yield of stilbene was constant and increased exponentially indicating
stability of the product and catalyst up to 200 minute reaction time. The Pd-TiO2 catalysts
recorded selectivity values of up to 100% and high yields of up to 83% as
calculated from GC relative areas. In addition Pd-TiO2 was
effectively eliminated from the final products by filtration as compared to
unsupported Pd(OAc)2 and
Pd/C catalysts. Similar trends were observed for C-C coupling of
iodobenzene with n-butyl-acrylate.
Work in progress is geared
towards mechanistic studies to understand the nature of very active metal
species in solution. Similarly, related work
is underway on fabrication and characterization of gold nanoparticles and other
metal oxide nanostructured supports such as ZnO and ZrO2 and their
application to Heck and Suzuki C-C coupling reactions.
Back to top