
Back to Table of Contents
43948-B1
Stereospecific Intramolecular Carbenoid Insertions on Furanose Platforms as a Route to Branched-Chain Sugars, C-Glycosides and Fused Heterocycles
Peter Norris, Youngstown State University
The use
of enantiomerically pure carbohydrates for the
synthesis of other complex molecules relies on the availability of
methodologies that are compatible with the associated functionality present in
the saccharide substrate. To expand the available methods we are
investigating the application of carbenoid insertion
chemistry for the formation of carbon-carbon bonds on conformationally
restricted furanose platforms. The method holds great promise for the stereospecific formation of chiral
heterocycles, including those related to C-glycosides, and the frameworks of
several classes on natural products.
Recently we have constructed diazoesters
attached through O-3 of several D-xylofuranose
platforms and studied their Rh(II)-catalyzed decomposition chemistry, which results in
remarkably different outcomes in each case.
For an O-3 linked compound in which O-5 is blocked by a trityl protecting group, the major process involves regioeselective intramolecular
C-H insertion into the C-2 – H-2 bond of the xylofuranose
ring to generate fused bisfuran products. The reaction is stereospecific
with respect to the xylose ring, however there is
little selectivity seen in the formation of the new chiral
center alpha to the ester carbonyl. The
structures of both diastereomers have been solved by
X-ray diffraction which proves the stereochemical
relationships between the two fused furan rings. Decomposition of an O-3 linked diazoester featuring an azide
group in place of O-5 results in quite different products in which the azido group becomes involved in the chemistry. The major isolated products are an oxazepine, the product of an intramolecular
cyclization process, and a 14-membered macrocyle which is the outcome of the intermolecular cyclization variant.
The structures of both of these compounds have been solved by X-ray
diffraction (Figures 1 and 2). The
mechanism for this remarkable, and seemingly unprecedented, transformation is
currently under investigation. Our
preliminary work on sugar-derived diazoesters led to
the discovery of a new one-pot synthesis of glycosyl azides from the corresponding lactol
precursors and we have expanded the scope of this reaction to non-carbohydrate
alcohols and subsequently to sequential one-pot processes such as
1,2,3-triazole synthesis. Displacement
of azide from p-acetamidobenzenesulfonyl
azide by the alkoxide gives
an intermediate sulfonate ester, and subsequent displacement
on an alkyl or acyl halide with azide
anion affords the alkyl or acyl azide. Reaction progress is monitored conveniently
using IR spectroscopy since each of the azide species
involved has a distinct absorbance frequency.
Isolation of the potentially dangerous azide
product is unnecessary and subsequent reaction, for example with terminal
alkynes in the presence of a Cu(I) catalyst, is proving to be a promising route
to 1,2,3-triazoles in one flask from the precursor alkyl or acyl
halide. These reactions are also
amenable to the application of microwave heating.
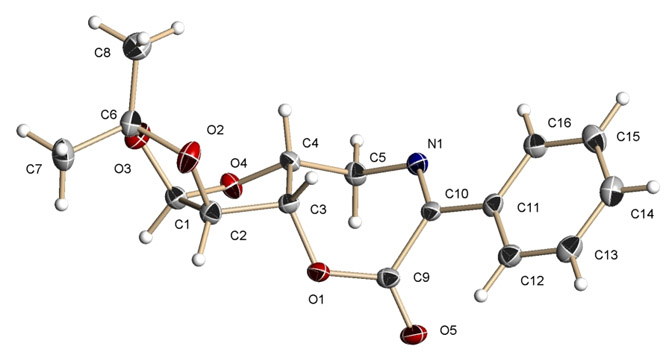
Figure 1.
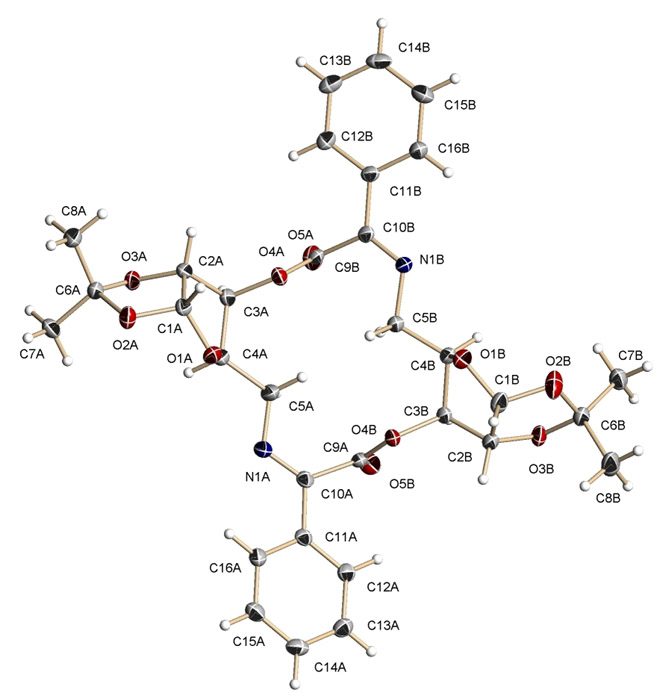
Figure 2.
Back to top



