47143-G7
The Effects of Polydispersity on the Melt Phase and Aqueous Solution Phase Behavior of ABA-Triblock Copolymers
Overview. Block copolymers and related polymer surfactants combine two or more chemically dissimilar homopolymer blocks into a single macromolecule. Neat block copolymers self-assemble in the melt-phase and in the solution-phase to produce well-defined nanostructured materials. These materials find widespread applications as thermoplastic elastomers, pressure-sensitive adhesives, and as non-ionic surfactants for cosmetics and enhanced oil recovery. The phase behavior of block copolymers comprised of two monomers having narrow molecular weight distributions has been well studied. Production of monodisperse block copolymers requires demanding synthetic conditions that contribute considerably to their cost, and the resulting materials are known to often exhibit unfavorable processing characteristics that limit their utility. Systematic experimental explorations of the influence of molecular weight polydispersity on the phase behavior of block copolymers and their related surfactants are scarce. Therefore, we are examining the fundamental effects of selectively incorporating polydisperse center segments into ABA-type triblock copolymers on their melt-phase behavior and their dilute solution-phase behavior. Our initial studies focus on two ABA-type triblock copolymer model systems: (1) poly(styrene-b-1,4-butadiene-b-styrene) (SBS) and (2) poly(ethylene oxide-b-1,4-butadiene-b-ethylene oxide) (OBO). These systems both offer opportunities to study the melt-phase behavior of weakly segregated block copolymers, while the latter system enables studies of the dilute solution behavior of polydisperse amphiphiles. Our preliminary results indicate that the quiescent melt-phase behavior of polydisperse SBS triblock copolymers is dramatically different from that of their monodisperse counterparts.
Melt-phase
behavior of SBS triblock copolymers. ROMP-CT
of 1,5-cycloctadiene in the presence of 1,4-dibromo-2-butene catalyzed by
Grubbs'-type catalysts produces a,w-dibromo-poly(1,4-butadiene) with Mw/Mn = 2.0. Subsequent atom transfer radical polymerization
(ATRP) of styrene in the presence of a copper(I) catalyst produces SBS triblock
copolymers. Selective catalytic degradation of these triblock copolymers with
OsO4/H2O2 demonstrates that the poly(styrene)
end blocks have polydispersities Mw/Mn ² 1.15. By this methodology, one
graduate student and one undergraduate student supported by ACS PRF funding over the last 12 months have produced a
library of 40 fully characterized polymers having a range of compositions and
molecular weights.
Polydisperse
SBS triblock copolymers microphase separate into ordered structures having
variable degrees of long-range order as evidenced by small-angle x-ray
scattering. Temperature dependent SAXS analyses of the ordered melts at
symmetric compositions (fstyrene = fbutadiene = 0.5) reveal large increases
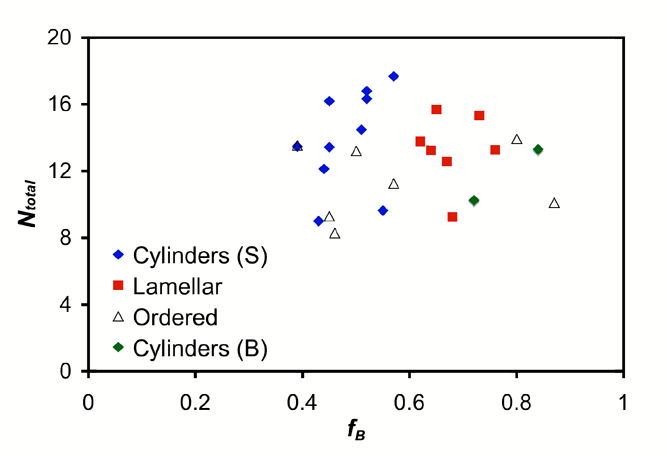
Figure 1. A tentative phase diagram for polydisperse SBS triblock copolymers. in the order-disorder transition
temperatures, indicating substantial stabilization of the polydisperse melts.
In contrast to monodisperse SBS triblocks, the polydisperse melts adopt a
cylindrical morphology at symmetric compositions instead of the typically
observed lamellar structure. Synchrotron SAXS further demonstrates that some of
the samples exhibit coexistence of microphase separated morphologies, e.g. a mixture of lamellae and hexagonally packed
cylinders. A tentative phase map for these triblocks copolymers is given in
Figure 1, which indicates that the phase boundaries for polydisperse melts
shift dramatically and the intermaterial dividing surfaces curve toward the
polydisperse segment. Current work focuses on corroborating the results of SAXS
analyses using transmission electron microscopy to provide real-space images of
these ordered structures.
Solution-phase
behavior of OBO triblock copolymers.
Employing a variant of the synthetic strategy described above, ROMP-CT provides
ready access to a,w-dihydroxy-poly(1,4-butadiene) with Mw/Mn = 2.0. Anionic ring-opening polymerization of ethylene
oxide initiated from the hydroxyl termini yields OBO triblock copolymers, with
monodisperse O blocks. We are currently optimizing the synthesis of these
materials for future studies of their melt-phase behavior and aqueous solution
phase behavior. We anticipate that these triblocks will exhibit different
properties from their monodisperse counterparts, which may lead to novel
rheological modifiers that are readily produced using scalable chemistries.
 General
Synthetic Strategy. Our synthetic strategy
relies on well-known methods in chain transfer ring-opening metathesis
polymerization (ROMP-CT) to produce difunctional telechelic polymers with a
polydispersity Mw/Mn = 2.0. By installing functionalities at the chain ends
that enable the controlled/"living" polymerization of a second monomer,
ABA-triblock copolymers are readily produced in which the A end blocks are
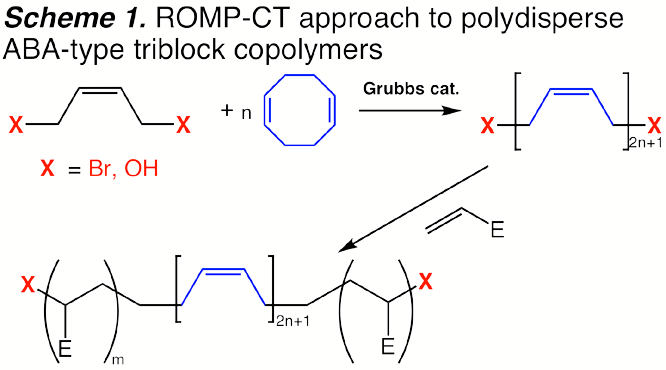
monodisperse (Mw/Mn ² 1.15) and the center B block is
polydisperse (Scheme 1). Polymers derived from this methodology in which the
center block is poly(1,4-butadiene) from ROMP of 1,5-cyclooctadiene offer an
advantage over other systems of interest: selective degradation of the
polydisperse poly(1,4-butadiene) block enables direct analysis of the molecular
weight and polydispersity of the monodisperse outer blocks. The complete
parameterization of these polymers enables quantitative comparisons with
theoretical predictions.
General
Synthetic Strategy. Our synthetic strategy
relies on well-known methods in chain transfer ring-opening metathesis
polymerization (ROMP-CT) to produce difunctional telechelic polymers with a
polydispersity Mw/Mn = 2.0. By installing functionalities at the chain ends
that enable the controlled/"living" polymerization of a second monomer,
ABA-triblock copolymers are readily produced in which the A end blocks are
monodisperse (Mw/Mn ² 1.15) and the center B block is
polydisperse (Scheme 1). Polymers derived from this methodology in which the
center block is poly(1,4-butadiene) from ROMP of 1,5-cyclooctadiene offer an
advantage over other systems of interest: selective degradation of the
polydisperse poly(1,4-butadiene) block enables direct analysis of the molecular
weight and polydispersity of the monodisperse outer blocks. The complete
parameterization of these polymers enables quantitative comparisons with
theoretical predictions.