44172-AC10
Next Generation Models for Predicting the Shape of Solution-Grown Organic Crystals in the Presence of an Additive
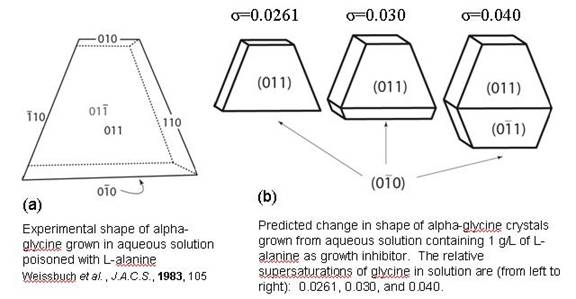
Our research deals with the use of tailor-made additives as growth inhibitors for manipulating the shape of organic crystals. It is well known that foreign molecules (additives or impurities) influence the shape of crystals. These molecules can be divided into two broad categories: molecular and macromolecular. Assuming the crystallizing solute has a molecular mass of no more than 300-500, a macromolecular additive or impurity could be a protein, polymer, or peptide, while a molecular additive could be a surfactant or fatty acid, etc. Another important type of molecular impurity or additive is one which is structurally related to the solute, here referred to as being an imposter. As the name suggests, an imposter is a molecule that is practically identical to the crystallizing solute molecule; these types of molecules are relevant to crystallization because they can be reaction by-products formed during the synthesis of the solute. There are many reports in the literature that describe the effect of such additives or impurities on the shape or growth of crystals Previous models of this effect have assumed that adsorbed immobile impurities decrease the perpendicular growth rate of a crystal face by reducing the velocity (rate of solute uptake) at an edge; specifically, the immobile impurities partition the edge into a collection of segments and the growth of those segments whose length is less than or equal to some critical length is arrested, thus decreasing the edge velocity. However, we show that under dilute imposter conditions this is not expected, and we demonstrate that the rate of solute uptake at an edge is, on average, unchanged. Rather, we argue the distances travelled by edges during the first turn of a growth spiral on a crystal face are increased, thereby decreasing the density of steps across the face and reducing the perpendicular growth rate of the crystal face. The details of our model are reported in Sizemore and Doherty (2008). We have applied the model to the crystallization of alpha-glycine grown from water in the presence of the imposter L-alanine. The predicted crystal shapes of are shown in Fig. 1(b). The left-most crystal in Fig. 1(b) reflects a prediction at a relative supersaturation of 0.0261, which is the largest value for which our model predicts the total stoppage of growth; in other words, the dead zone of relative supersaturation is predicted here to be in the interval (0 to 0.0261). The specificity of binding and the effect of L-alanine and other chiral amino acids on the growth of alpha-glycine is well-documented (Weissbuch et al. 1983, Weissbuch et al. 1988). Fig. 1(a) is a representation of an alpha-glycine crystal nucleated and grown in the presence of L-alanine by Weissbuch et al. (1983); as can be seen, only growth in the +b direction occurred because of the stereospecific binding of L-alanine at the (02(bar)0) face (it is noted that the 010 families in Fig. 1(a) are equivalent to 020 when corrected for systematic absences in the x-ray diffraction pattern). Moreover, Weissbuch et al. (1988) report growing seeds of alpha-glycine in the presence of S-leucine dissolved at levels ranging from 0.25% to 1.5% by mass of the solute. At low relative supersaturations (approx. 0.025), they observed the growth rate of (02(bar)0) was reduced by a factor ~3 relative to the (020) face. In the calculations here, L-alanine was assumed present at 0.5% by mass of glycine in the solution and, at relative supersaturation = 0.040, the growth rate of (02(bar)0) was reduced by a factor of 3 relative to the (020) face. In this case, it appears that the model yields predictions qualitatively and quantitatively in keeping with experimental results. Fig. 1. Experimental and predicted shapes of alpha-glycine crystals grown from water in the presence of imposter L-alanine. References Sizemore, J.P. and M.F. Doherty, “A New Model for the Effect of Molecular Imposters on the Shape of Faceted Molecular Crystals,” J. Crystal Growth, submitted (2008). Weissbuch, I., L. Addadi, Z. Berkovitch-Yellin, E. Gati, S. Weinstein, M. Lahav and L. Leiserowitz, “Centrosymmetric Crystals for the Direct Assignment of the Absolute Configuration of Chiral Molecules. Application to the Alpha-Amino Acids by Their Effect on Glycine Weissbuch, I., L. Addadi, L. Leiserowitz and M. Lahav, “Total Asymmetric Transformations at Interfaces with Centrosymmetric Crystals: Role of Hydrophobic and Kinetic Effects in the Crystallization of the System Glycine/Alpha-Amino Acids,” JACS, 110(2), 561 (1988).