46311-AC6
Kinetics, Reaction Mechanisms, and Relative Stabilities of Heterocyclic Ring Molecules in Ionized Environments
Hydrocarbon ions and neutrals are known to be important in the chemistry of interstellar gas clouds, planetary atmospheres (such as the planetary satellite Titan) and in combustion in supersonic ram jets. Some of the species detected are ring hydrocarbons implying that other rings could be present. Possibilities are both homo-and hetero-cyclic and aromatic and non-aromatic molecules such as pyridine, C5H5N; pyrimidine, C4H4N2; piperidine, C5H11N; dioxane, C4H8O2; benzene, C6H6; cyclohexane, C6H12 (all six-membered rings) and the five-membered rings, pyrrole, C4H5N; pyrrolidine, C4H9N; furan, C4H4O. A central ion in the chemistry of these media is CH3+ which can react with the rings in very different ways (proton transfer, charge transfer (both dissociative and non-dissociative)), hydride abstraction and association. The mechanisms of these reactions are expected to be variable depending on the availability of pi electrons in the rings and of lone pairs on the N and O heteroatoms with the breaking of the aromaticity in some cases. In studying these reactions, it was a surprise to see that association is competitive with hydride abstraction and also with proton transfer and charge transfer when these are exothermic, since they are considered to proceed on every collision. Association, except in the case of furan, occurs when there are pi electrons in the ring and does not occur when there are not. From theoretical molecular dynamic calculations in the literature for the reaction of CH3+ with benzene, the mechanism is considered to initially proceed by attraction of the ion to the plane of the pi electrons. This fast forming complex then rapidly isomerizes to a stable sigma complex where the CH3+ attaches to a carbon in the ring displacing the H atom. That proton transfer is only minor whereas charge transfer is competitive with the other channels could be because, for proton transfer, a localized charge site is required whereas charge transfer could be long range and delocalized.
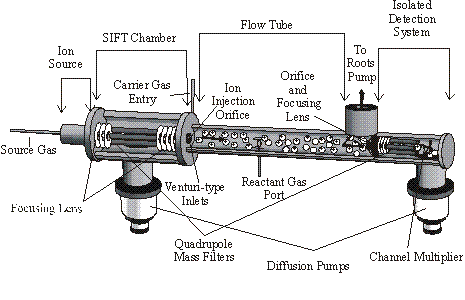
The reactions were studied experimentally using a Selected Ion Flow Tube, see Figure 1.
Figure 1. Here CH3+
ions are created by low pressure electron impact on methane with the CH3+
being selected in a quadrupole mass filter and
injected into a flow tube containing helium at 0.5 Torr. The helium then carries the ions to a
downstream quadrupole mass filter/ion counting
system, by the action of a Roots blower pump.
In the intervening region, neutral reactant ring compounds are
introduced into the flow. Ionized
reaction products are also detected by the downstream counting system and rate
constants and product ion distributions are determined in the standard
way. All of the ring compounds used in
this study are liquids and were introduced into the gas phase by diluting the
vapor in helium at a pressure less than the saturated vapor pressure.
For the six-membered
rings, when N atoms are introduced into the aromatic ring (C5H5N
and C4H4N2) the aromaticity
is retained and the association and hydride abstraction channels are large. When the ring is not aromatic (C5H11N
and C6H12), there is no association, but hydride
abstraction remains large, presumably because of the availability of a large number
of H atoms. In the five-membered aromatic rings containing N heteroatoms,
association and hydride abstraction are consistently small or non-existent even
though the aromaticity is maintained. As expected, for O substitution where there
are no pi electrons, these channels remain at zero. Note that when O and N atoms are introduced
into the rings, lone pairs become available introducing additional sites for
reaction and other reaction channels.
In these
studies, the isomeric form of the products is not known, e.g., is the product
of the CH3+ + C6H6 reaction protonated toluene or some other isomeric form. To investigate this sort of question, a crude
SID (surface induced dissociation) detector has been constructed in the
expectation that different isomeric forms will dissociate in different ways. If this is successful, an upgraded form with
be constructed. A heated neutral source
has also been constructed with which lower vapor pressure species from liquids
and solids can be introduced into the flow tube. This is waiting for a suitable time to be
tested.
In an earlier study, it was
established in reactions of O+ with C5H5N that
C4H4+ + HCN was the only channel (and dominant
with Ar+, N2+, N+
and Kr+). Similarly for C4H4N2
only C3H3N+ + HCN resulted. By microscopic reversibility, charged C5H5N+
and C4H4N2+ should be produced by
the reverse associations if there are no activation barriers. Preliminary studies of the former reaction
have shown rapid association and efforts are underway to determine whether ring
closure occurs in the formation of the product.
If this occurs, then this will be an interesting mechanism and could be
the source of the ionized pyridine in the Titan ionosphere.