

46222-B1
Design of Nitrogen, Phosphorus and/or Sulfur-based Catalysts for the Enantioselective Hydrosilylation of Prochiral Ketones
Synthesis and Application of Thioureas as Ligands
Asymmetric hydrosilylation of arylketones catalyzed by transition metals with chiral ligands is a useful synthetic route to optically active alcohols.1 Polymethylhydrosiloxane (PMHS) has been used as efficient hydrosilylating reagent with chiral zinc diamine-based catalysts in the asymmetric reduction of prochiral ketones.1,2 Recently, considerable attention has been focused on the development of thiourea ligands, which form air- and moisture stable catalysts precursors.3
Herein, we report the preparation of chiral thioureas 1 – 8, from C2-symmetric 1,2-diamines and isothiocyanates affording dithioureas 1 – 4 and monothioureas 5 – 8 in 79 – 98% yield, after purification by column chromatography on silica gel [petroleum ether:EtOAc; 12:1]. (Figure 1).
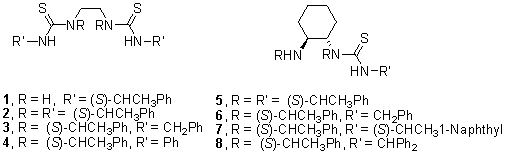
Figure 1
The chiral N,S-thiourea ligands 1 – 8 were used in the enantioselective hydrosilylation of acetophenone, in the presence of Et2Zn and PMHS as hydride source. Yields were measured after column chromatography on silica gel [hexane:EtOAc; 10:1] as eluent. The enantiomeric excesses and configuration were determined by HPLC with a chiralcel OD column. Best result was achieved with monothiourea 5 (Scheme 1).
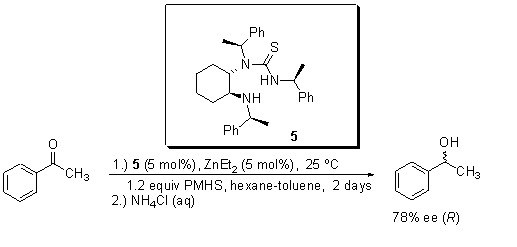
Scheme 1
In conclusion, we prepared new chiral N,S-thioureas ligands 1 – 8. Low to good enantiomeric excesseses (up to 78% ee) were achieved in the asymmetric hydrosilylation of acetophenone. The evaluation of thiourea 5 as ligand in the asymmetric reduction of other prochiral ketones is in progress.
Synthesis of Bis(Sulfonamido)Diol
A multidentate bis(sulfonamide) diol ligand incorporating trans-1,2-diaminocyclohexane was reported by Walsh et. al.4 We later probe the nature of the chiral diamine backbone by preparing an analogous ligand based on trans-1,2-diaminocyclopentane.5
Herein, we report the synthesis from (R,R)-trans-11,12-diamino-9,10-dihydro-9,10-etanoanthracene 9 (Figure 2).6-7 Then, diamine 9 is used as starting material for the preparation of bis(sulfonamide)diol 10 in the presence of (S)-camphorsulfonyl chloride (Figure 2).
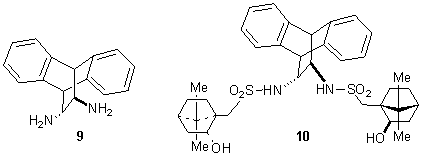
In conclusion, we prepared enantiopure bis(sulfonamide)diol 11 (7% overall yield) and optimization of the reaction conditions as well as the application as asymmetric catalyst are in due course. Furthermore, we will use (R,R)-trans-11,12-diamino-9,10-dihydro-9,10-etanoanthracene 9 as starting material for the synthesis other ligands.
Final Remarks
We have learn more about the structure-reactivity relationship of thioureas 1 – 8 as ligands in the asymmetric hydrosilylation. Also, we begin the work in the preparation of (R,R)-trans-11,12-diamino-9,10-dihydro-9,10-etanoanthracene 9 and bis(sulfonamido)diol 10, which will have further applications in this project.
In the development of the experimental work, the undergraduate students have been actively involved. They worked as a team with other students at my lab. Besides, the undergraduate students became aware about their possibilities for future graduate studies in chemistry. They also learn more about Organic Synthesis and Green Chemistry.
It has been a great opportunity to involve undergraduate students in doing research. All of us have grown by the interaction with each other. The financial support of the ACS-PRF has been unvaluable for the development of this project.
References
(1) Riant, O.; Mostefai, N.; Courmarcel, J. Synthesis 2004, 18, 2943-2958.
(2) Mastranzo, V. M.; Quintero, L.; de Parrodi, C. A.; Juaristi, E.; Walsh, P. J. Tetrahedron 2004, 60, 1781-1789.
(3) Karamé, I. M.; Tommasino, L.; Lemaire, M. J. Mol. Catal. A Chem 2003, 196, 137-143.
(4) García, C.; LaRochelle, L. K.; Walsh, P. J. J. Am. Chem. Soc.2002, 124, 10970.
(5) Anaya de Parrodi, C. ; Walsh, P. J. Synlett 2004, (13), 2417-2420.
(6) Allenmark, S.; Skogsberg, U.; Thunberg, L. Tetrahedron: Asymmetry, 2000, 11, 3527-3534.
(7) Fox, M. E.; Gerlach, A.; Lennon, I. C. ; Meek, G.; Praquin, C. Synthesis 2006, (19), 3196 – 3198.