
Back to Table of Contents
45586-AC3
Tunable Control of Magnetic Properties in Transition Metal Indenyl Complexes
Timothy P. Hanusa, Vanderbilt University
This research has as its goal the
investigation of the synthesis, structures, and magnetic properties of
(indenyl)metal complexes that display adjustable changes in spin state. Ultimately
we aim to create new classes of magnetically active compounds, including
charge-transfer molecular magnets that would be the foundation for data storage
applications, and compounds whose spin state could control their behavior as
synthetic reagents.An extensive study of methylated
bis(indenyl)Cr(II) complexes of Cr(II) has been completed. These compounds
display spin states that are strongly dependent on the orientation of the
indenyl ligands; i.e., compounds with staggered ligands are high spin, with
four unpaired electrons (S = 2), whereas complexes with eclipsed ligands display thermally
induced spin-crossover behavior. The origin of this behavior was identified as
a consequence of particular symmetry interactions between the ligands and metals.Although it was expected that the
substitution of a large number of electron-donating methyl groups would favor
low-spin complexes, it was a surprise to learn that their specific location on
the indenyl ligands has a strong effect on the spin states; methyl groups on
the six-membered benzo ring appear to be more effective in stabilizing a
low-spin ground state than are those on the five-membered ring. Extensive
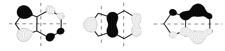
density functional theory (DFT) calculations were conducted at the TPSSh/6-31+G(d)
level, which traced the origin of this effect to the influence that
substitution on the benzo ring has on the HOMO-2
(pi-3) orbital of the indenyl anion (see rightmost Figure below). The
energy of the pi-3 orbital is raised by 0.30 eV relative to the unsubstituted
anion. This ultimately has the effect of lowering the energy of the molecular
HOMO, which favors the low-spin state. The tunability of the magnetic
properties of bis(indenyl)metal complexes with site-specific ligand
substitution has implications in the construction of charge transfer salts. It
more broadly hints at the significant electronic (as distinct from steric)
modifications that could be exploited with indenyl ligands that are used in
polymerization catalysts.
We
have made an extended push into manganese indenyl chemistry. The rationale for
this is our previous isolation of the air-stable charge transfer salt,
[(1,2,3-Me3C9H4)2Fe][DCNQ] (DCNQ =
2,3-dicyanonaphtho-1,4-quinone), which was found to be a magnetically ordered
at low temperature. The incorporation of complexes with spin-crossover behavior
into charge-transfer salt magnets could potentially induce new types of
magnetic behavior, including thermal hysteresis. Cationic Mn(III) complexes,
containing d4 metal centers, would be isoelectronic with neutral
Cr(II) species, and might display analogous orientation-dependent spin
crossover behavior. Bis(indenyl)Mn(II) compounds would be the logical
precursors to the Mn(III) species. Although
the unsubstituted complex (C9H7)2Mn is
unknown, we have prepared and crystallographically characterized methylated
versions, which can be directly compared to their chromium(II) counterparts.
Initial investigations of their chemistry have been spectacular. For example,
the yellow (2,4,7-Me3C9H4)MnCl(thf) complex
forms blue solutions when cooled under a nitrogen atmosphere; the blue color is
discharged upon purging the solution with argon. All indications are that a
dinitrogen complex is formed at low temperature, the first that we are aware of
that involves Mn(II). The compound also reversibly binds dihydrogen, forming
purple solutions at low temperature; a dihydrogen (or dihydride) complex is
probably involved. The reaction with nitrogen is also displayed by the
bis(indenyl)Mn complexes (2-MeC9H6)2Mn and
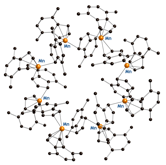
(2,4,7-Me3C9H4)2Mn. Despite an
intriguing octomeric structure (see Figure below), the related (4,7-Me2C9H5)2Mn
complex does not bind dinitrogen. It appears that some methylation of the five-membered
ring (and possibly a spin state change) is critical for activating the
(indenylę)Mn complexes toward nitrogen/hydrogen binding.
We are continuing to accompany these
synthetic, crystallographic, and magnetic studies with computational investigations,
mainly employing DFT methods. Calculations will used to examine the comparative
energy changes occurring on the binding of gases and in the Mn(II)/Mn(III)
complexes incorporated into charge-transfer salts.
This research has put the PI into
contact with the magnetic materials community, which has expanded his
background in synthesis and structural characterization in new directions. It
has also lead to an increasing involvement in computer modeling and
calculations, and to a greater appreciation of the way in which computational
results can illuminate experimental findings and suggest new directions in
synthesis. It has lead to several presentations at regional and national
meetings. The graduate student involved in the research has had to expand his
repertoire of synthetic and computational knowledge, and to learn to
communicate with scientists who are not in his immediate field of chemistry. He
has presented his work at a national ACS meeting, and a second-year student that
he is training will discuss the work at a regional ACS meeting later this year.
Back to top





