

43980-G5
Atomically Resolved Reaction Dynamics Involving Catalytically Active, Surface Supported Clusters
While the
majority of our work and funding support for this project has been dedicated to
the establishment of capabilities in our group to pursue experiments in
catalytic behavior of metallic particles, we have explored further external
support from the National Science Foundation and the Department of Energy. Both single investigator and multi-PI
proposals have been submitted over the past year to continue work on selective
and tuned catalytic interfaces. Of
particular note is the burgeoning collaboration with a computational group at
NCSU led by Marco Buongiorno Nardelli. We
have explored a number of research directions where we can combine density
functional theory calculations with our experiments to interrogate reaction
energetics and mechanistic pathways. It
our intention to pursue further funding with the ACS-PRF with a New Directions
grant to investigate low dimensional materials for selective catalysis, where the
role of size and surface defect type and composition can be utilized in the
catalytic recycling of carbon dioxide. Recently, we have been able to make significant
progress in the study of metallic island growth on ferroelectric surfaces. Specifically we have been studying the growth
morphology for Au deposited on to single crystalline, uniformly poled lithium
niobate (LiNbO3). This
ferroelectric has a very high surface charge density (~70 microCoulombs/cm2)
and has been studied by our group for selective adsorption through
electrostatic anchoring of polarizable molecules. Our attention has now turned to the study of
metal islands and ultra-thin films for the study of surface reactivity,
specifically the possibility of controlling surface reactions though electric
field driven changes in the local density of states at the interface. These metal
layer experiments are being performed using AFM, LEED, Auger electron
spectroscopy, TEM, as well as computational modeling. The funds from
this program have been invaluable to our research group with respect to establishing
a new area of work especially in directions we had not initially anticipated. We have also been able to use this support to
begin collaborative work involving high performance simulations and predictions
in concert with scanning tunneling microscopy. We anticipate that we will make even further
progress in the study of catalytic activity for thin metal films, metal
clusters, and atomic chains that have substantially different electronic and
therefore chemical bonding attributes compared to their bulk counterpart. ADDIN
EN.REFLIST 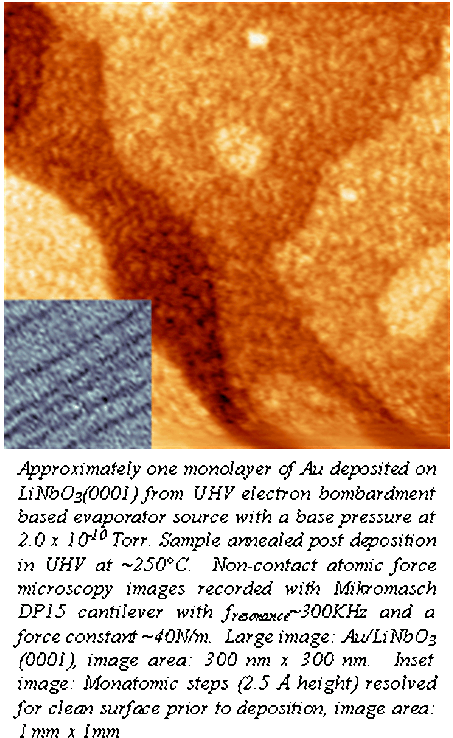 Opportunities exist for dictating and monitoring the influence
of confined geometry, reduced dimensionality, and anisotropic electronic
effects on surface reactivity, especially catalytic reactions. The degree to which an interface can be tuned
chemically whether through the substrate or a supported thin film determines
the scope for nanostructured media to be synthesized with enhanced reaction
selectivity and efficiency. Over the
past two years, funds from the ACS-Petroleum Research
Foundation Type G award have been used to achieve a number of different project
goals. The
first has been to purchase key equipment, supplies, and materials for
positioning experiments to be performed. An electron beam evaporator for the controlled
deposition of various metals on metallic oxides in ultra-high vacuum has been
purchased and was delivered in January 2008.
This piece of equipment has been added to our UHV-STM/AFM system which
is currently under construction. Clusters
of varying size of key catalytically active metals including Pt and Cu, from
isolated adatoms to large, nanometer sized, multilayer facets, can be deposited
onto catalytic supports such as graphite (0001) and metallic oxide thin films
such as alumina and barium oxide. Single crystalline alloy samples like NiAl(110) and
Ag(111) have likewise been acquired using this award. Summer support for the PI has been used
during year 2 to support project goals as well as expand to other heterogeneous
catalytic systems. Funds from this award
have also been used to purchase chemical reagents and liquid cryogen for
operating low and variable temperature scanning probe microscopes.
Opportunities exist for dictating and monitoring the influence
of confined geometry, reduced dimensionality, and anisotropic electronic
effects on surface reactivity, especially catalytic reactions. The degree to which an interface can be tuned
chemically whether through the substrate or a supported thin film determines
the scope for nanostructured media to be synthesized with enhanced reaction
selectivity and efficiency. Over the
past two years, funds from the ACS-Petroleum Research
Foundation Type G award have been used to achieve a number of different project
goals. The
first has been to purchase key equipment, supplies, and materials for
positioning experiments to be performed. An electron beam evaporator for the controlled
deposition of various metals on metallic oxides in ultra-high vacuum has been
purchased and was delivered in January 2008.
This piece of equipment has been added to our UHV-STM/AFM system which
is currently under construction. Clusters
of varying size of key catalytically active metals including Pt and Cu, from
isolated adatoms to large, nanometer sized, multilayer facets, can be deposited
onto catalytic supports such as graphite (0001) and metallic oxide thin films
such as alumina and barium oxide. Single crystalline alloy samples like NiAl(110) and
Ag(111) have likewise been acquired using this award. Summer support for the PI has been used
during year 2 to support project goals as well as expand to other heterogeneous
catalytic systems. Funds from this award
have also been used to purchase chemical reagents and liquid cryogen for
operating low and variable temperature scanning probe microscopes.