
Back to Table of Contents
42824-B6
Spectroscopic Studies of Cyclic Enones in Triplet Excited States
Stephen Drucker, University of Wisconsin (Eau Claire)
1. Measurement and analyses of singlet-triplet spectra of 4H-pyran-4-one (4PN) and
4-cyclopentene-1,3-dione (4CPD). We have recorded the T1 (n,π*) ← S0 spectra of these molecules to examine the
delocalized effects of π* ← n excitation. We measured the
4PN and 4CPD spectra at room temperature, using phosphorescence excitation (PE)
and cavity ringdown (CRD) detection, respectively. During the third year of the
grant period (2007-08), we completed the vibronic analyses of the 4PN and 4CPD
spectra, published our results on 4PN, and reported the 4CPD spectrum at the
63rd International Symposium on Molecular Spectroscopy. Figure 1 shows the
PE spectrum of 4PN in the region of the T1← S0 origin band. Most of the Δv=0
sequence bands are assigned to ν18 (ring bending) and ν13
(ring twisting). The positions of the sequence bands imply that the frequencies
for ring bending and twisting are 126 cm-1 and
269 cm-1,
respectively, in the T1 state. It is interesting to compare changes
in the 4PN ring frequencies for S1 (n,π*) ← S0 vs. T1 (n,π*) ← S0 excitation. The
drop in ring-twisting frequency is 32% for the S1 excitation
(R. D. Gordon and W. K. C. Park, Can. J. Chem., 71:
1672, 1993), which is very similar to the 34% reduction observed for T1.
So for both of the excited states, the effects of π* ← n promotion clearly extend to the highly conjugated system
of ring atoms. However, these effects are not exactly the same in the two
excited states: the S1 ring-bending frequency is 145 cm-1,
barely reduced from its ground-state value of 149 cm-1.
This contrasts with a drop of 15% in the T1 state. Thus in 4PN, as
we found for 2-cyclopenten-1-one (2CP) previously, the chromophore differs in
subtle but discernable ways for singlet vs. triplet excitation. For both
molecules, delocalization into the ring appears to be more extensive in the
triplet case, as evidenced by changes in the ring-bending potentials.
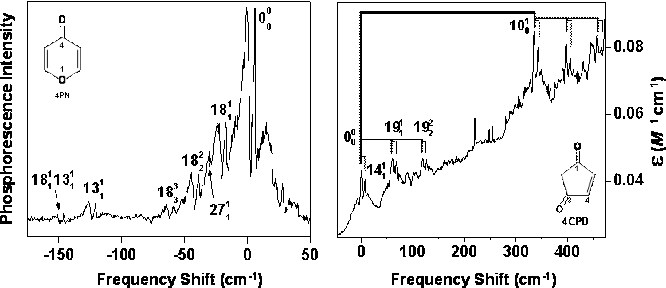
Figure 1:
T1 (n,π*) ←S0
spectra of 4PN (left) and 4CPD (right). Frequencies are relative to the origin
bands at 27,292 and 20,541 cm-1,
respectively. We have also studied the
π* ← n excitation
in 4CPD, a constitutional isomer of 4PN. Figure 1 shows the CRD spectrum of
4CPD near the T1 (n,π*) ←S0
origin band. The assigned sequence structure indicates that the ν19
(ring bending) fundamental frequency is 60 cm-1 higher in the T1
excited state than in the ground state. Each of the ν19
sequence bands has a satellite observed 7 cm-1 higher in frequency. We
assign the satellites as combinations of the main bands with the ring-twisting
transition 1411. As with the other cyclic enones, the S1
and T1 states of 4CPD show differing effects of π* ← n excitation. Whereas S1
and T1 show the same 60% increase in ring-bending frequency
compared to the ground state, only the S1 state has a substantially
increased ring-twisting frequency: the ground-state frequency is 239
cm-1 (R. A. Back and
R. D. Gordon, J. Mol. Spectrosc., 204: 85, 2000), which
changes to 304 cm-1 in S1 (ibid.) (a 28% increase), but only 246 cm-1 in T1 (a 3%
increase).
To
summarize our results on the three planar molecules 2CP, 4PN, and 4CPD, the
antibonding effects of the π* ← n excitation extend to the
ring atoms in cases of both singlet and triplet excitation. However, the triplet
state, in its own specific way for each molecule, shows a more extensive
weakening of the forces responsible for ring planarity.
2. Construction of
jet-cooling apparatus. In
grant year 3, we also completed construction of an apparatus that will allow us
to record electronic spectra under jet-cooled conditions. This approach will
eliminate nearly all the vibronic hot bands and thereby improve the precision
in the vibronic band assignments of the current and future spectra. The jet
cooling will also permit rotational contour analyses. The new apparatus
consists of a vacuum chamber (six-way cross) pumped by a diffusion pump
that contains an internal water-cooled baffle. The chamber is equipped with a
current loop-actuated valve (Jordan TOF Products, Inc.) that can produce very
short (60 µs) pulses under choke flow conditions. Shown in Figure 2 is the
intense jet-cooled fluorescence excitation spectrum of the test molecule CS2,
coexpanded with 2 atm helium carrier gas. The relative line intensities
indicate an effective rotational temperature around 5 K.
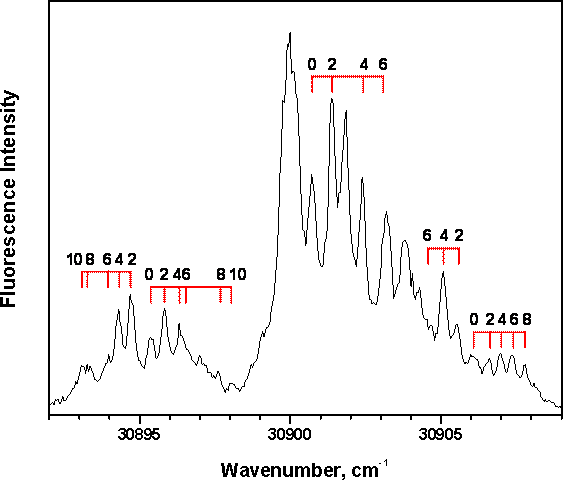
Figure
2: Jet-cooled fluorescence excitation spectrum of CS2.
The dye laser resolution was approximately 0.2 cm-1. The spectrum
shows the 000 (K′=0) ← 0000
band of the 1B2
(1Δu) ←1Σg+ system. The band is strongly perturbed by
numerous triplet levels, giving rise to several sets of P and R branches. Tie
lies near resolved branches indicate J″
assignments determined previously (K. Nishizawa et al., J. Chem. Phys., 100:
3394 (1994)). Odd J″ levels are
missing due to nuclear spin statistics.
Back to top





