44682-G4
Functional Zippers to Organize Synthetic and Biological Polymers
Nature makes assemblies of discrete size and shape that catalyze reactions, recognize molecules with high specificity, and direct translocation. Our proposal targeted the synthesis of controlled supramolecular structures using urea-urea based zippers as an organizational unit. In this second year of the grant we have advanced our research on three fronts: 1) We measured the association contains of a number of rigid bis-urea macrocycles in solution. 2) We made progress towards the synthesis of the bis-acetylene models and will soon study their assembly in solution by NMR and IR. 3) We synthesized leucine zipper peptide models. Attempts at the synthesis of the same peptides with citrullenes substituted for all leucines resulted in truncated sequences, as the urea side chain reacted with the active ester on the backbone to form a macrocycle. These truncations have led us to evaluate different urea protecting groups for the synthesis.
It is fundamentally important to chemistry, biological chemistry, and material science to understand how non-covalent forces guide the conformations of monomers and assemblies to form ordered, semi-ordered or 'folded' materials. We probed the limits of urea organizing motif by studying the assembly of small models 2-6 in both solid state and in solution. We examined the effects of rigidity and flexibility both the structure of assembly formed and on the association constants in a range of solutions. We found that the additional of heteroatoms, which might compete as hydrogen bond acceptors, did not disrupt assembly unless the system was quite flexible. We gained valuable experience in analyzing the structure of these aggregates in solution. We estimated the aggregate size as primarily dimers and trimers by PSGE NMR diffusion studies. This aggregation behavior was further followed using 1H NMR and IR by taking spectra at varying oligomer concentrations. This work led to the recent submission of a manuscript to J. Org. Chem. "An examination of the structural features that favor columnar self-assembly of bis-urea macrocycles."
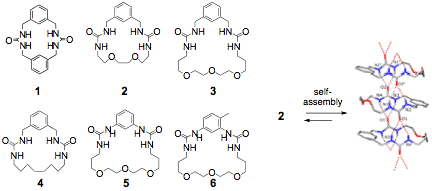
Figure 1: Small model ureas 2-6 were used to evaluate the limits of the urea assembly motif both in solution and in the solid-state.
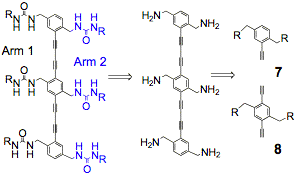
Our proposal targets the synthesis of discrete finite structures using urea-urea based zippers as an organizational tool. We have experience making 'infinite' extended arrays with urea macrocycles. The challenge is to use this motif to form well-defined assemblies of finite size and shape. We focused on the synthesis of rigid rod phenyl bis-acetylenes that are preorganized to self assemble into dimers, trimers in solution (Figure 2). Our efforts to synthesize targeted the synthesis of single and double-sided oligomers that are programmed to assemble into well-defined structures through strong urea hydrogen bonds.
A) B)
|
|
|
Figure 2: A) Functional zippers bring together separate polymer chains to form discrete mesoscale assemblies. B) Double sided (two armed) trimers can be synthesized from building blocks 7 and 8. Single sided (one armed) derivatives are synthesized from 7 and 8 each having only one R group.
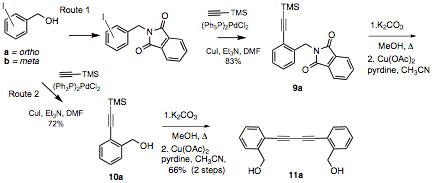
Two summer students evaluated the feasibility of two routes for bis-acetylene synthesis (Scheme 1). Sonagashira coupling of both ortho or meta iodobenzyl alcohol gave coupled products in good to excellent yields. Subsequent Mitsunobu reaction yielded the phthalimide 9a. Unfortunately, deprotection and coupling of the terminal acetylene under several different conditions gave poor yields of the bis-acetylene. We therefore, moved to introducing the phthalimide later (Route 2, Scheme 1). It appears that the order of introduction of the urea group is important, as TMS-deprotection of the 10a gave the bis-acetylene 11a in 66 % yield (for 2 steps) and we are currently optimizing conditions for the Mitsunobu reaction. Subsequent deprotection of the phthalimide followed by treatment with an isocyanate would complete the synthesis. We are poised to finish these syntheses in the next six months.
Scheme 1: Evaluation of methods for bis-acetylene synthesis
In summary, funding from this generous type G Petroleum Research Fund grant enabled us to pursue a new line of research in stabilize mesoscale urea assemblies. We developed tools and techniques to analyze these systems. We ventured into new areas of bis-acetylene preparation and peptide synthesis. These new techniques were applied to our ongoing projects in porous crystals as confined reaction environments and gave important contributions to three 2008 publications as well as one submitted manuscript. We plan to use these findings as preliminary results to apply for grants at NSF and NIH. In addition, over the last two years this grant supported the development of scientists including graduate student Mahender B. Dewal, undergraduates Eleanor Gillette (Univ. of Pittsburgh) and Shada McCurry (SUMR supplement) and aided Dr. Sharon Strickland (Assistant Professor at Converse College) who returned to research. The SRF fellowship provided leverage for gaining funds from Converse for outfitting her own laboratory.