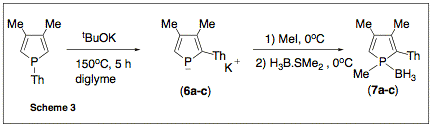44734-AC1
Polyphospholes and Hetero Analogs
The essence of
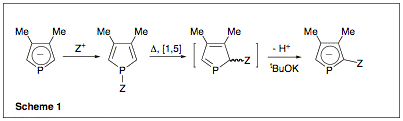
our project is to use our original phosphole functionalization technique as
depicted in scheme (1)1 to prepare new families of
phosphole-heterole oligomers for optoelectronic applications. After several
attempts, we were able to devise an access to
original
phosphole-silylene-thiophene chains. These results have been described in our
previous report. For practical applications, this synthesis proved to be
somewhat difficult to upscale. We thus decided to look for other types of
conjugated phosphole oligomers both original and accessible via our
functionalization technique. We have found such a family of products and
started to investigate its optoelectronic properties in collaboration with our
UCR colleague, professor Christopher J. Bardeen.
Synthetic results Three groups
have definitively established the fact that conjugated thiophene-phosphole
oligomers display an impressive potential for the manufacture of organic light
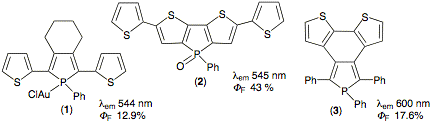
emitting diodes (OLED's). A typical product developed by the group of Réau is
phosphole gold complex (1).2 Dithienophosphole (2) is representative of the work of Baumgartner.3
Finally, Matano has recently developed another type of thieno-annellated
phosphole represented by (3).4
These results have
served as a basis for our new work. We first
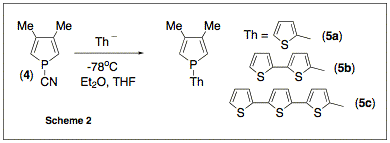
synthesized several polythienyl-substituted phospholes as shown in scheme (2).
Then, we
investigated the thermal behavior of (5a-5c) in the presence of KOtBu. The
choice of the solvent proved to be critical. At 150oC in diglyme, a
clean reaction takes place to give the expected phospholides (6a-6c) (Scheme 3).
The phospholides
were characterized as their 1-methylphosphole borane complexes. Compound (7b) was also characterized by
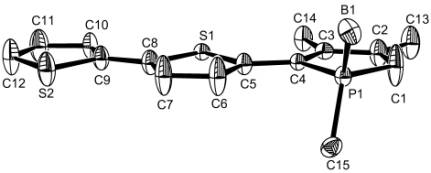
X-ray crystal structure analysis (fig. 1). The most characteristic features of
the structure are the perfect coplanarity of the three rings, the high
dissymmetry of the phosphole ring (P1-C1 1.762(3) vs P1-C4 1.810(3) Ā) and the
short bridge bonds between the rings (C4-C5 1.443(4) and C8-C9 1.452(4) Ā).
Figure
1: X-ray crystal structure analysis of (7b). Optical
properties Table (1)
summarizes the quantum yield and lifetime measurements for compounds 7b and 7c
as well as those of terthiophene and quaterthiophene.
TABLE 1
labs(nm) lfl(nm) tfl (ns) Ffl Dm 7b 384.3, 382.6 469.5,476.4 0.100 0.016 3.8 7c 410.7, 409.1 502.0, 511.0 1.22 0.178 4.4 terthiophene 354.5, 354.3 421.6, 420.5 0.188 0.060 0.4 quaterthiophene 392 478 0.49 0.18 - Table 1. Spectroscopic parameters for
oligothiophenes and phosphole analogs made in this work. For the peak absorption and
fluorescence wavelengths labs and lfl, the first value is
in toluene and second is in CH2Cl2.
An important
difference between the thiophene and phosphole compounds is the fact that the
absorption and emission are shifted to longer wavelengths in the phosphole
compounds. The shift in wavelength, along with differences in the fluorescence
lifetimes, suggest that the excited states in 7b and 7c have different
electronic characteristics from those of pure oligothiophenes. To further probe the nature of the
excited states, we varied the polarity of the solvent. Excited states with more charge
transfer character should show fluorescence spectra that shift to lower
energies in more polar solvents.
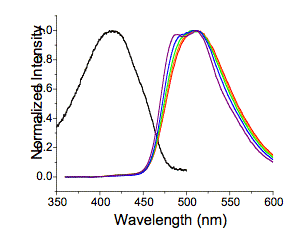
That is exactly what we see in the phosphole analog 7c (Figure 2).
Figure 2: Black = 7c absorption in 100% toluene. The absorption in 100% CH2Cl2
is the same. Purple = fluorescence
in 100% toluene; blue = fluorescence in 75% toluene:25% CH2Cl2;
green = fluorescence in 50% toluene:50% CH2Cl2; orange
fluorescence in 25% toluene:75% CH2Cl2; red =
fluorescence in 100% CH2Cl2. Note that terthiophene shows at most a 1 nm shift under the
same conditions. From these data,
using the Lippert-Mataga formula, we can extract the change in dipole moment
upon photon absorption (Dm), which
gives an indication of the charge transfer nature of the excited state.5
For terthiophene, the change in dipole is only 0.6 Debye, while for 7b and 7c, the changes are 3.8 and 4.4 Debye respectively,
with about 10% error in all values.
The larger values for Dm
confirm that the phosphole analogs have excited states with greater charge
transfer character than the oligothiophenes.
<>Future work Contrary to the
other phosphole-thiophene conjugated oligomers (1)-(3), our compounds
display a highly dissymmetrical structure and a huge charge transfer in the
excited state. We can modulate this transfer by replacing the terthiophene by a
longer oligothiophene, changing the substitution at phosphorus and grafting a
functional substituent at the a'
position of the phosphole ring using the chemistry of scheme (1).
References
1) 2) 3) 4) 5)