Reports: DNI954912-DNI9: The Role of Phase Behavior in Fischer-Tropsch Product Distributions: Supercritical Fluid Studies
Michael Timko, PhD, Worcester Polytechnic Institute
Objectives. The main objective of this work was the study of the influence of phase behavior on heterogeneously catalyzed chemical reaction. This progress report is an update on phase behavior and catalytic activity studies.
Phase Behavior & Reaction Engineering. Initially, we intended to study the Fischer-Tropsch synthesis (FTS) reaction as a model for examining the interaction between phase behavior and heterogeneous catalysis. Delays in implementation of safety protocols – specifically for CO – and realization of the challenges of delivering high precision flows of high pressure gases (CO and H2 at 100 bar) to the flow reactor led us to instead use select two different model systems, both in near or supercritical fluids: 1) dodecane cracking and 2) ethanol dehydration. As with FTS, solid acid catalyzed reactions are extremely important in the oil and gas industry; in fact, fluidized catalytic cracking may be the single most important application of heterogeneous catalysis. And, like supercritical FTS, liquid phase reactions catalyzed by solid acids have many interesting features: 1) most importantly for this work, the reactions are heterogeneous, and with potential phase behavior limitations and 2) operation in or near the phase separation boundary may have benefits in terms of process intensity and catalyst lifetime.
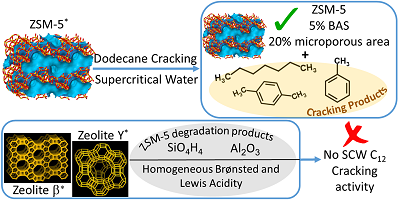
A main area of work has been confirming the heterogeneous nature of zeolite-catalyzed cracking of dodecane in supercritical water. We selected ZSM-5 as previous studies had suggested it to have catalytic activity in supercritical water. However, no previous study had investigated if ZSM-5 was the actual catalyst. Therefore, we set out to test the heterogeneous activity as rigorously as possible. First, we verified that addition of ZSM-5 to a dodecane-water mixture results promotes dodecane cracking. Interestingly, zeolites HY and b did not. Characterization tests revealed that ZSM-5 alone retained crystallinity in supercritical water. Therefore, we concluded that HY and b completely collapse, either destroying or reducing reactant access to acid sites. Next, we confirmed that ZSM-5 is reusable in supercritical water, a necessary requirement for heterogeneous activity. Finally, we comprehensively tested all potential degradation products for activity, including: silicalite, alumina, silicic acid, and activated carbon (to simulate coke). Of these, silicic acid and activated carbon exhibited some activity, but not sufficient activity to explain ZSM-5 result. Lastly, we tested the supernatant liquid recovered from a ZSM-5 catalyzed dodecane cracking experiment. Again, we found the supernatant liquid to lack activity. Figure 1 summarizes our findings. Catalysis Today recently accepted the work for publication, and we anticipate it to be published in a special issue in Spring 2018.
Figure 1. Heterogeneous activity of ZSM-5 in supercritical water.
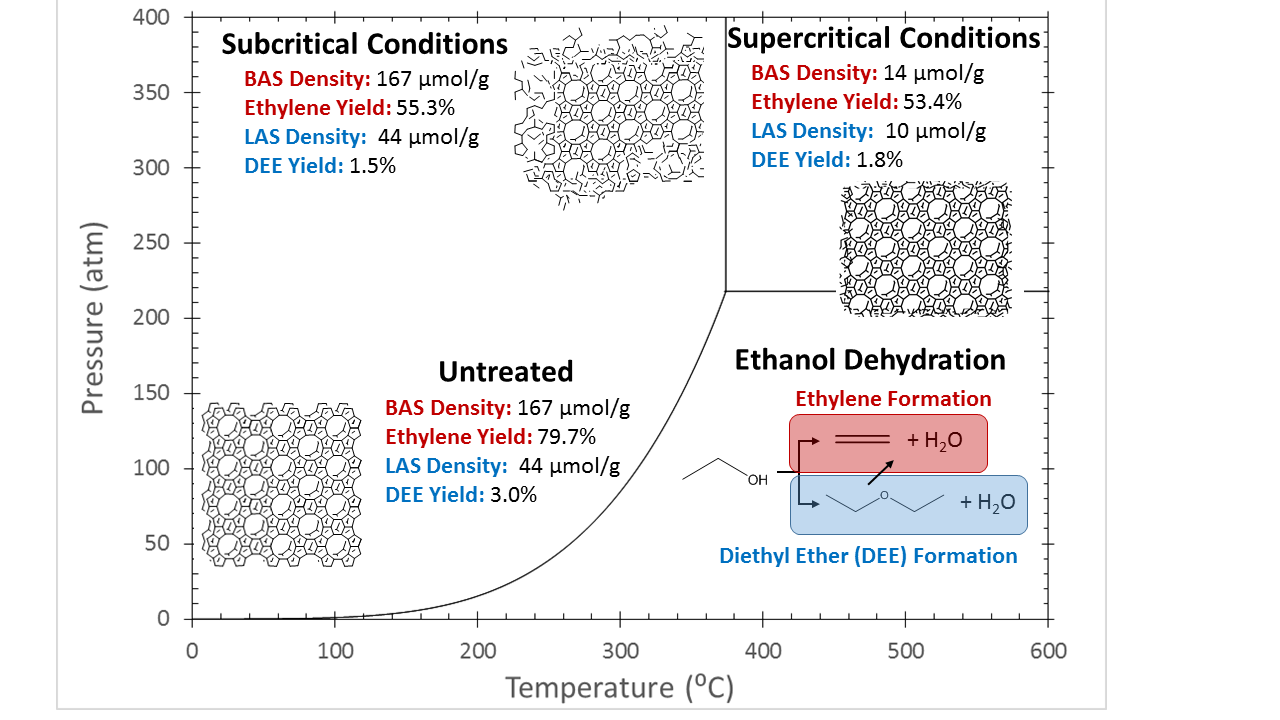
A second main area has been an in-depth study of ZSM-5 stability in supercritical water. Through a combination of many different techniques, including XRD, SEM, TEM, Al and Si NMR, FTIR, and reaction probes, we now recognize that ZSM-5 degradation occurs via parallel de-alumination and de-silication. De-silication proceeds via a two-step process. First, amorphous surface layers a exfoliated. Second, the crystalline framework is attacked, from the outside in. De-alumination appears to follow a similar mechanism as under steaming conditions, albeit at enhanced rates. Figure 2 summarizes the main results. Interestingly, we find that ZSM-5 framework is more stable in dense water at temperatures greater than the water critical point. We attribute this finding to the role of polarized transition states on framework desilication. These findings provide a clear path forward to understand the effect of water on zeolite activity. We anticipate submitting the first full manuscript on this work to Nature Catalysis in Spring 2018.
Figure 2. De-silication and de-alumination of ZSM-5 in supercritical water.
The third area of study has been ethanol dehydration. Ethanol dehydration is simple, acid catalyzed reaction that again has the key features of phase behavior and heterogeneous catalysis. We have found that operation in liquid phase greatly decreases activity, with the exception being at low space velocities. We are now investigating the effect of water on liquid-phase and vapor-phase activity at low space velocities and expect to finalize the study in summer 2018.
Continuing Work. The PRF opened up many avenues for future work. In fact, the fundamental science proved to be much richer than anticipated. We now realize that water plays a complicated role in hydrocarbon phase behavior, zeolite stability, activity, and internal pore diffusion. We anticipate following up this work with support from industry, DOE, NSF, and others.
Products. To date, the award has resulted in the following products:
· A.R. Maag, M. Timko, “Liquid-Phase Stability of ZSM-5: Role of De-Alumination and De-Silication”, presented at AIChE (Salt Lake City, UT), November 2015, New England Catalysis Society (Providence, RI), May 2016, and Gordon Conference (Holderness, NH), June 2016.
· A. Zaker, M. Timko, “Catalytic Cracking of Dodecane in Supercritical Water”, New England Catalysis Society (Providence, RI), May 2016.
· M. Timko, “Hydrothermal Stability Strategies for Solid Acid Catalysts”, University of Connecticut (invited lecture), May 2016.
· M. Timko, “Promise and Pitfalls of Liquid Phase Catalysis”, Tufts University (December 2017), New York University (April 2018), and Catalysis Gordon Research Conference (June 2018), invited talks & presentations.
· A Zaker, P. Guerra, G. Tompsett, M. Timko, “Catalytic Cracking of Dodecane in Supercritical Water” AIChE Presentation, November 2016, identified as “best presentation of the session”.
· M. Timko, “Materials and Chemicals from Renewable and Waste Carbon Sources: Reaction Engineering and Spectroscopy”, International Symposium on Chemical Reactor Engineering, June, 2016.
· A Zaker, P Guerra, Y. Wang, G. Tompsett, X. Huang, J. Bond, M. Timko, Zeolite Catalyzed Cracking of Dodecane in Supercritical Water: Lewis and Brønsted Acidity, Catalysis Today, accepted 2018.