Reports: UNI555164-UNI5: Investigation of Methane Direct Dissociation and Partial Oxidation on Vanadium Surfaces
Heather L. Abbott-Lyon, PhD, Kennesaw State University
Year 2 Progress Report
Methane is an abundant resource within the United States. Converting methane into partially oxidized compounds such as methanol and formaldehyde, which are important feedstock compounds in the chemical industry, is highly desirable. Current methods for methane conversion to liquid hydrocarbons involve an indirect two-step process: 1) syngas (CO + H2) is produced via oxidation of methane and 2) a Fischer-Tropsch reaction is used to create a hydrocarbon. While a direct conversion method would be preferred, it is difficult to perform partial oxidative dehydrogenation rather than complete oxidation to CO2 because of the reaction thermodynamics. Vanadium oxide catalysts are known to be effective for the partial oxidation of methanol to formaldehyde. We hypothesize that a methoxy intermediate, similar to the one observed for CH3OH to CH2O conversion, may be important in the partial oxidation of methane to methanol. Using experimental and theoretical methods to investigate the activity of bare and oxygen pre-covered vanadium surfaces, the Abbott-Lyon Lab (ALL) will investigate the direct conversion of methane to methanol. In particular, methane dissociative sticking coefficients will be measured as a function of independently varied gas and surface temperatures. These non-equilibrium measurements will be analyzed using a microcanonical unimolecular rate theory (MURT) to extract the reaction threshold energy (E0) and other characteristics of the transition state such as the contribution from the surface.
In order to perform the dissociative sticking experiments, a heated effusive molecular beam doser was designed and built during Year 1 of this award (Figures 1 and 2). Additionally, the MURT code was updated for CH4/V(100) and sticking coefficient predictions were made assuming a range of conditions based on other CH4/metal systems that have been studied.
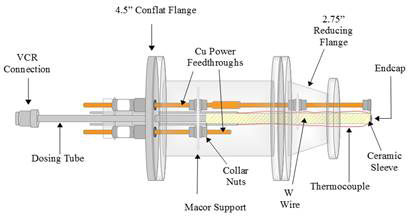
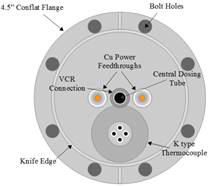
Figure 1. Schematic diagrams of the variable temperature effusive molecular beam, viewed from the top (left) and from the rear (right), were created in LibreCAD.
Figure 2. Photographs of the variable temperature effusive molecular beam built in the Abbott-Lyon lab. The image on the left shows the bare doser, while the image on the right shows it ready to be attached to the UHV chamber.
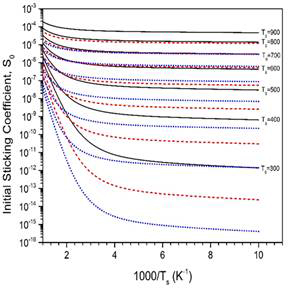
Figure 3. MURT predictions of the dissociative sticking coefficient as a function of reaction threshold energy (E0). For these calculations, 2 surface oscillators were assumed and a low frequency of 200 cm-1.
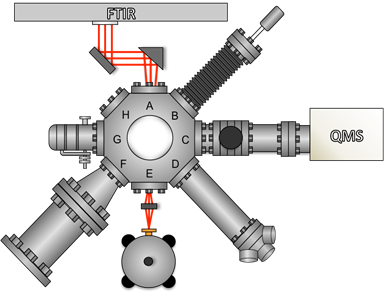
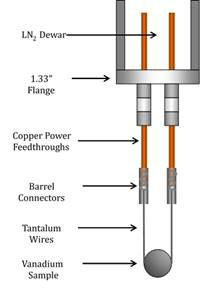
There have been a few challenges during Year 2. After installing the doser on the ultrahigh vacuum chamber, an electrical short was detected. While repairing the heated doser, the UHV system in the ALL underwent upgrades, which were required for and funded by another project in the lab. This new system will make the planned CH4/V(100) experiments easier to perform. For example, a new Ertl-style sample holder with improved heating and cooling capabilities was designed and built. This will contribute a smaller background signal to mass spectrometry measurements. Illustrations of the new UHV chamber and sample holder are shown in Figure 4.
Figure 4. Illustrations of the new UHV chamber in the Abbott-Lyon laboratory (left) and the new sample holder (right) are shown. Labeled flanges on the UHV chamber correspond to: A) incident infrared beam, B) Knudsen cell, C) quadrupole mass spectrometer, D) electron gun (for sputter cleaning), E) reflected infrared beam, F) effusive molecular beam, G) variable leak valve, and H) window.
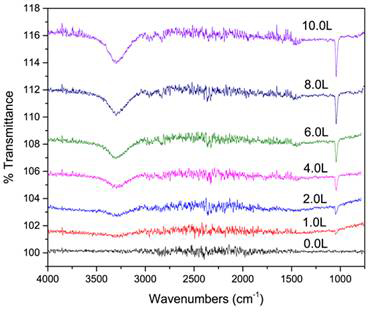
Reflection-absorption infrared spectroscopy (RAIRS) experiments were performed to benchmark our experiments with previous measurement made for CH3OH on V(100). Our experiments show a sharp feature at 1042 cm-1 corresponding to the C-O stretch and a broad feature ~3200-3300 cm-1 corresponding to the O-H stretch of methanol. (n.b., Challenges with the purge for the external RAIRS optics resulted in signal-to-noise ratios that are less than ideal between 1500-2800 cm-1.)
Figure 5. RAIRS spectra of CH3OH adsorbed onto V(100) at Ts = 100 K are shown as a function of dosage, where 1.0 Langmuir (L) = 10-6 Torr·s of exposure.
An unfortunate accident occurred at the beginning of summer 2017, which broke the electrical feedthroughs on the sample holder and scratched the vanadium single-crystal. Thus, the summer of Year 2 was spent repairing the sample manipulator and getting the sample repolished. It is now ready to be mounted in the UHV chamber again. While this accident delayed progress with the experiments, it was a valuable learning experience for the undergraduate students involved.
To date this award has directly impacted five undergraduate students and one masters student. Undergraduates were involved in: 1) designing and constructing the heated effusive molecular beam, 2) performing MURT calculations on the methane/vanadium system, and 3) modifying the sample holder to improve the heating and cooling for dissociative sticking and RAIRS measurements. The students involved in the project learned to use LibreCAD (a free autoCAD program), to spotweld, to operate and maintain ultrahigh vacuum equipment, and to present their research findings. The three undergraduate students who worked on this project during Year 1 were all upperclassman, who have now graduated. One of these students is currently pursuing his doctorate in chemistry at Georgia Tech. The MS student working on this project successfully defended his thesis in May 2016 and is now a research technician with a local company. His thesis is available through Digital Commons. The two undergraduate students who started on the project in Summer 2017 are underclassman with several semesters remaining before graduation. In addition to providing research support for students, this ACS PRF award has also provided summer support for PI Abbott-Lyon, allowing research rather than teaching to be pursued during the summer months.