Reports: UNI655479-UNI6: Mechanistic Details of Iron Based Fischer-Tropsch Catalysts: Ion Trap Experiments on Model Active Sites
Joshua J. Melko, PhD, University of North Florida
Introduction
This research program studies the Fischer-Tropsch reactions on gas phase iron based systems, aiming to distinguish the preferred mechanism(s) and identify the reaction intermediates involved. These outcomes are achieved by experimentally measuring thermodynamic and kinetic quantities via ion trap mass spectrometry in conjunction with experimentally vetted computational methods.
Results from Year 1
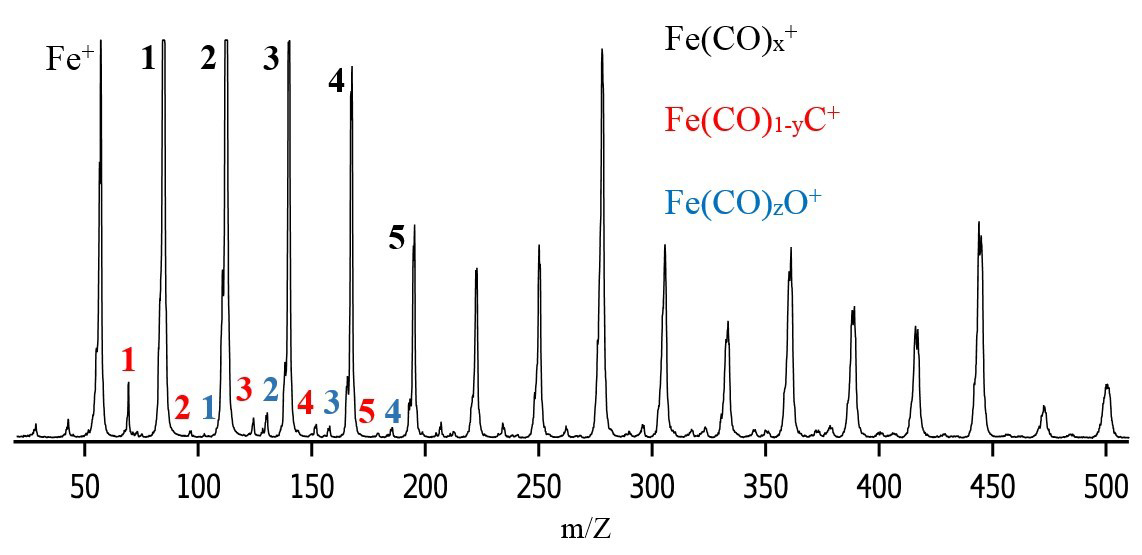
Aim one of the research program involves the synthesis and isolation of the iron based Fischer-Tropsch active sites in the gas phase. These synthesized species are then used in aim two of the research to conduct kinetic experiments with appropriate neutral molecules in an ion trap mass spectrometer. Aim one was accomplished in Year 1 as planned; an example of the ions created is shown below in a mass spectrum created and optimized by undergraduates.
Figure 1. Mass spectrum using a dilute Fe(CO)5 in helium mixture as the source gas.
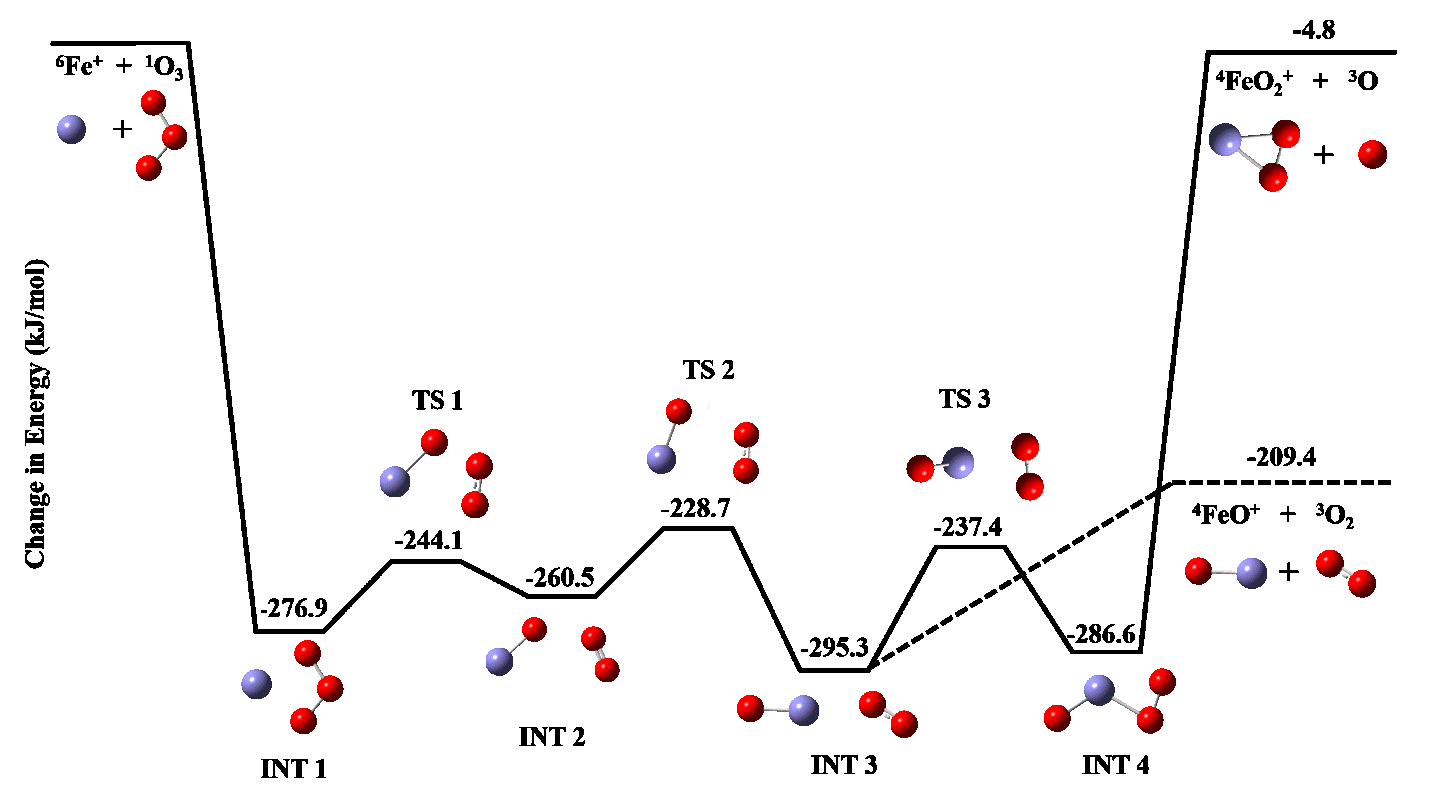
Also in Year 1, significant progress was made in the area of aim three, which employs computational chemistry to calculate structural and energetic parameters of the gas phase species. We used our methodology to investigate the reaction mechanism for Fe+ reactions with ozone, using experimental data from collaborators. As shown below in Figure 2, our calculations indicated the reaction proceeds through several intermediates and transition states before diverging into two product channels, corresponding to the FeO+ and FeO2+ ions. The lack of any significant kinetic barriers but a large difference in the exothermicity of the two reaction channels suggests the reaction should proceed efficiently, but produce mostly FeO+. This is in accordance with the experimental observations that the reaction proceeds at the collision rate, making only FeO+ without any FeO2+ formation.
Figure 2. Calculated reaction pathway showing intermediates (INT) and transition states (TS) for the reaction of Fe+ with ozone. Energies shown (in kJ/mol) are relative to the energy of the reactants.
Results from Year 2
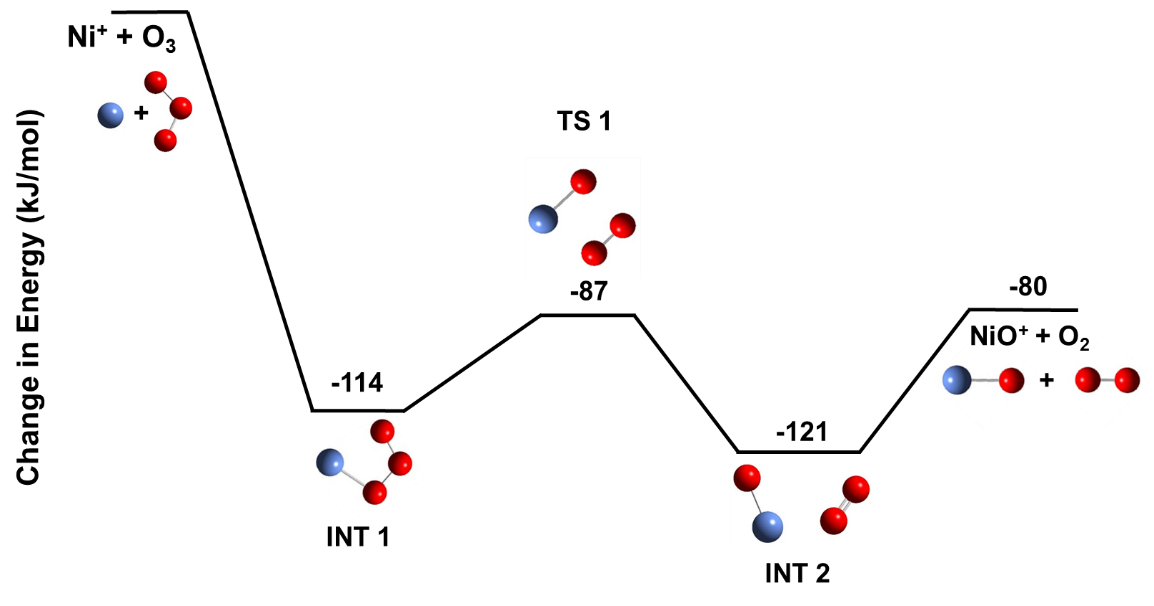
Transitioning to year two we aimed to publish the Fe+ + O3 results from year 1 and build on these results through study of similar systems. We were successful in publishing those results at the beginning of year 2 and quickly expanded our methodology and strategy to the analogous Ni+ + O3 system. There has been a long history of exploring nickel structures for use in Fischer-Tropsch systems. However, nickel induces methanation that limits the growth of long-chain hydrocarbons. The reasons for this are not well understood, and thus exploring the fundamental differences between nickel and iron reactivity was important to us. Figure 3 below shows that the reaction of nickel with ozone begins with the same type of encounter structure (INT 1) experienced by iron in Figure 2, but pivots through a transition state structure (TS 1) that is distinct from the iron case. The overall mechanisms is simpler and leads to only one set of products. Comparing Figures 2 and 3 allows one to see differences in the fundamental oxidation of these elements, which we find depends on the energy and bonding character of the transition metal’s low-lying orbitals.
Figure 3. Calculated reaction pathway showing intermediates (INT) and transition states (TS) for the reaction of Ni+ with ozone. Energies shown (in kJ/mol) are relative to the energy of the reactants.
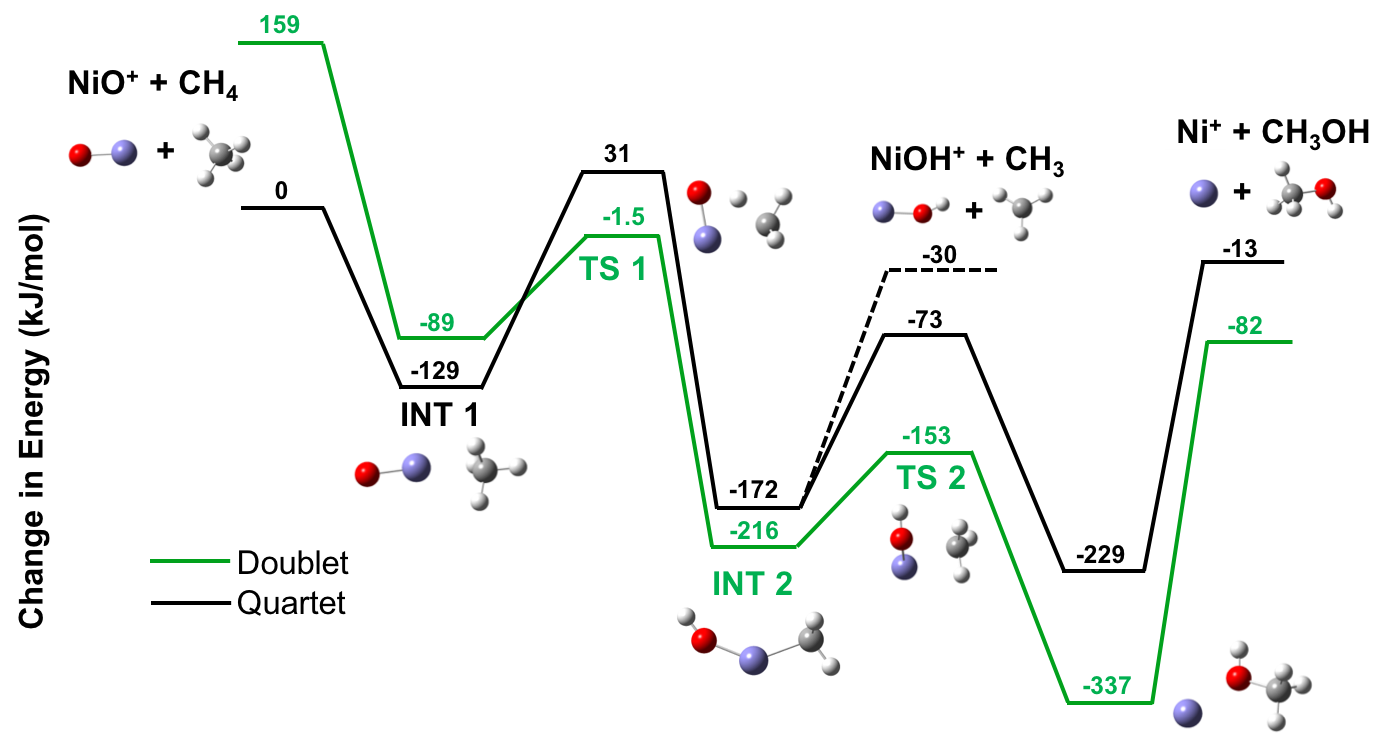
We have also expanded the study of nickel by examining the reaction of nickel oxide with methane. This provides a characterization, albeit a simple one, of the bonding motifs in methanation of nickel that prohibits its use as a Fischer-Tropsch material. The reaction mechanism is shown in Figure 4 below. We find two competing spin multiplicities (doublet and quartet) are present along the reaction coordinate. This allows the quartet reactants to bypass the energetic barrier at the first transition state (TS 1) via a spin flip to the doublet surface. This “two-state reactivity” has been observed in a number of other transition metal reactions, where it often governs the efficiency and product branching of the reaction.
Figure 4. Calculated reaction pathway showing intermediates (INT) and transition states (TS) for the reaction of NiO+ with methane. Energies shown (in kJ/mol) are relative to the energy of the reactants.
Impact on PI and Students
The support of the Petroleum Research Fun has kick-started the PI’s research program, leading to several presentations and a published manuscript. During the past year, two undergraduate students have worked on this project to accomplish the progress described in the “Results from Year 2” section above. In this short time, these two students have accounted for two poster presentations at a university symposium and two poster presentations at state conferences. In addition, these students have earned authorship on a manuscript currently in progress. This level of productivity would be impossible without the PRF funding, which has allowed these undergraduates to devote great chunks of time to research over the summer. The students learned tangible skills in working with high vacuum instrumentation, mass spectrometry, and computational chemistry, as well as the ability to analyze data, think critically, collaborate in a scientific environment, write manuscripts, and communicate scientific results. These skills have positioned them as competitive candidates for the next stage of their future scientific careers. One of these students is now applying to graduate school in physical chemistry, while the other is beginning her junior year.
The PI’s overall research productivity has also been enhanced by the support of the Petroleum Research Fund. This funding allowed the PI to concentrate on the training of students over the summer, it also provided materials and supplies for their experiments and the means to travel with undergraduates to disseminate the research. In addition, the PI has presented the PRF results at state and national conferences. The PI is now in a position to leverage these results into application for external funding. Specifically, the PI will target a Research in Undergraduate Institutions proposal to the National Science Foundation using results from this PRF program.