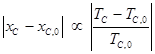Reports: UR1055088-UR10: Investigation of Pre-Transition Droplet Formation in Pseudobinary Liquid-Liquid Systems
J. Charles Williamson, PhD, Willamette University
This annual report encompasses the second year of grant activity. The broad goal of the project is to characterize the pre-transition droplet formation (PTDF) phenomenon that has been observed in pseudobinary liquid-liquid systems. Four objectives were delineated in the original proposal to meet this goal:
A. Assess the effect of extrinsic impurity doping on PTDF.
B. Explore the effect of isotopologue abundance percentages on PTDF.
C. Physically isolate pre-transition droplets and determine their composition.
D. Explore PTDF in liquid-liquid systems featuring Coulombic interactions.
Progress continued from the previous year on the first three objectives.
Objective A: In the previous year, preparatory work for extrinsic impurity doping tests was completed by measuring the phase behavior of the aniline + cyclohexane (ACH) liquid-liquid system. The first stages of the actual doping tests were completed this past summer. A list of possible dopants for the ACH system was compiled, and the candidate dopants were tested for miscibility with ACH, and for the rate of change of coexistence curve temperature Tcx with dopant concentration. The compound 2-picoline was selected as a dopant.
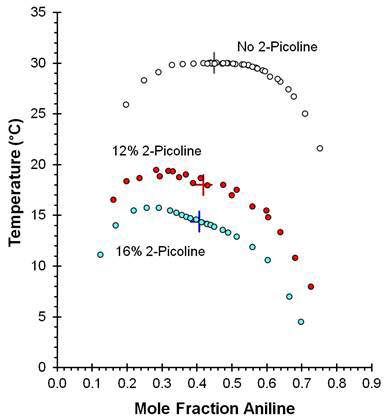
Two full sets of ACH doped with 2-picoline were prepared by the synthetic method. The stock aniline was doped with either 12 or 16 mole percent 2-picoline, while the cyclohexane was not doped. Each set thus had a dopant concentration that varied systematically with aniline composition. Dopant was added to the aniline because the aniline-rich solutions in the ACH system had been observed to exhibit PTDF. Figure One compares the coexistence curves measured for the ACH, AP12CH, and AP16CH systems. The noisy character of the AP12CH Tcx data was due to a mixing issue that was corrected before the AP16CH samples were prepared.
Figure One: Coexistence curve data for the (aniline + 2-picoline) + cyclohexane liquid-liquid system. White Circles: no 2-picoline; Red Circles: 12% 2-picoline; Blue Circles: 16% 2-picoline. Critical point locations are identified by the plus symbols.
The critical composition xC of each system was determined from analysis of 90° laser light scattering data, and the corresponding critical temperature TC was then interpolated from the coexistence curve. The critical temperature decreased –1.00 K per mole percent of 2-picoline dopant, and the critical composition in mole fraction aniline shifted –2.66×10–3 per mole per cent of 2-picoline dopant. According to Jacobs (Phys. Rev. A 1986, 33, 2605), the critical point in a perturbed liquid-liquid system follows the proportionality:
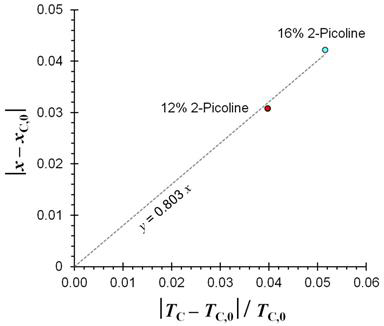
where (xC,0, TC,0) is the critical point in the absence of the perturbation. Figure Two presents a Jacobs plot for the APCH system, and the data are consistent with the Jacobs proportionality relation.
Figure Two: Jacobs plot for the (aniline + 2-picoline) + cyclohexane liquid-liquid system.
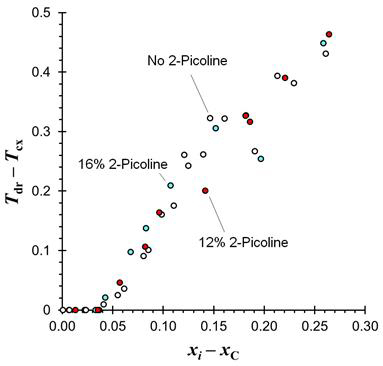
Pre-transition droplet formation was observed in the AP12CH, and AP16CH systems. The quantitative behavior of PTDF was identical to the PTDF behavior observed in the undoped ACH system. Figure Three shows the difference in temperature between the onset of PTDF and the bulk liquid-liquid phase transition, Tdr – Tcx, as a function of sample composition relative to the critical composition, x – xC. The onset and slope of all three curves appear to be the same, so the conclusion is that extrinsic doping has no effect on PTDF. PTDF behavior shifts with the shift in critical composition.
Figure Three: Pre-transition droplet formation behavior in (aniline + 2-picoline) + cyclohexane systems. White Circles: no 2-picoline; Red Circles: 12% 2-picoline; Blue Circles: 16% 2-picoline.
Objective B: The 1,2-propanediol + benzene and 1,2-propanediol + benzene-d6 liquid-liquid systems were characterized last year, and neither system exhibited PTDF. This past year several additional samples were made using 1,2-propanediol and an approximate 50/50 mix of benzene and benzene-d6. None of these samples exhibited PTDF. The conclusion is that isotopologue doping does not induce PTDF in systems that already do not exhibit PTDF.
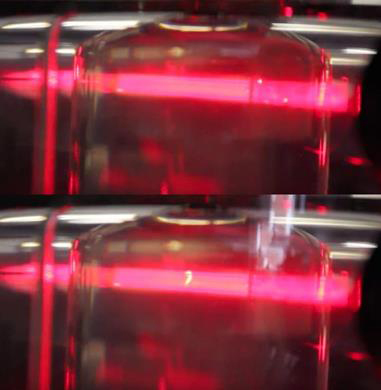
Objective C: The laser light scattering instrument designed for droplet extraction and constructed over the previous two summers was completed. PTDF was observed in preliminary tests on the instrument using an ACH sample (Figure Four), but droplet extraction was not attempted. In preparation for droplet extraction, a protocol was developed for quantitative determination of the composition of an ACH sample using a gas chromatography / mass spectrometry instrument.
Figure Four: (Top) Extraction ampule containing an aniline + cyclohexane mixture at a temperature above Tdr. (Bottom) The same system after cooling to a temperature between Tdr and Tcx. The pre-transition droplets are evident from the bright stripe in the center of the laser beam.
Impacts: In Fall 2016, two undergraduates who worked in Summer 2016 (MM, KR) presented a poster at the 25th Murdock College Science Research Conference. Their poster was an award winner in the “Analytical / Physical / Inorganic” category. One undergraduate (AW) worked on this project during the academic year, and results from this project were central to her Senior Thesis. She is now enrolled in a Master’s Industrial Internship program.
Two undergraduates (TK, MM) worked on the project in Summer 2017. Two undergraduates (MM, KR) each presented a poster at the 254th National American Chemical Society Meeting in Washington, DC. One undergraduate (MM) was recognized with an “Outstanding Student Poster Award” in the Physical Division. The PI gave a talk at this conference that included work supported by the grant.