Reports: DNI155732-DNI1: Hybrid Lewis Acid/Lewis Base Catalysts for Asymmetric Carbon-Carbon Bond Formation
Chris Dockendorff, PhD, Marquette University
Overview
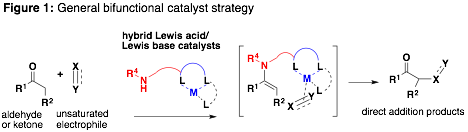
The catalysis arm of our research program is supported by this ACS PRF DNI grant for the discovery of bifunctional catalysts for the direct addition of aldehydes and ketones to alkynes. Additionally, this grant supported the completion of our early efforts towards the development of new asymmetric aldol catalysts. Our general strategy is outlined in FIG. 1.
Y2 Results
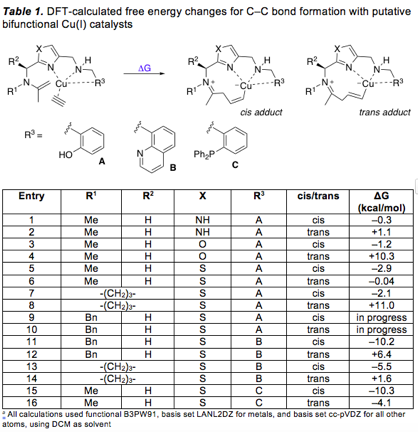
1. DFT calculations with bifunctional Cu(I) catalysts
We have now established workflows for DFT structural optimizations and free energy change calculations for our putative catalytic intermediates, to help us to prioritize our design ideas. Detailed calculations were performed with the precatalysts in Table 1 designed to bind Cu(I). Several trends are apparent from these calculations. First, the cis organocopper adducts are significantly lower in energy than the trans adducts, presumably to unfavorable ring strain in the macrocycle. Second, among several heterocyclic spacers studied, thiazole has consistently given the most favorable energies. Third, quinoline and diphenylphosphine groups are promising ligands at the "eastern" side of the catalysts, and have more favorable calculated energetics than the phenol (or phenolate) ligands. Quinoline-containing precatalysts have been synthesized (vide infra), but isolation of diphenylphosphine precatalysts in high purity has been challenging thus far.
2. Synthesis of precatalysts for use with Cu(I)
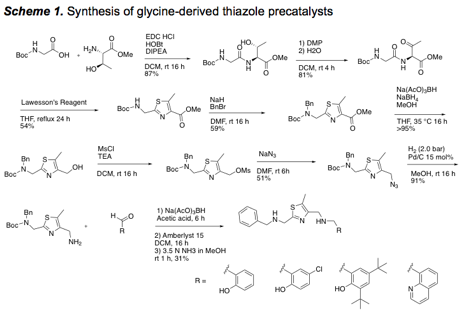
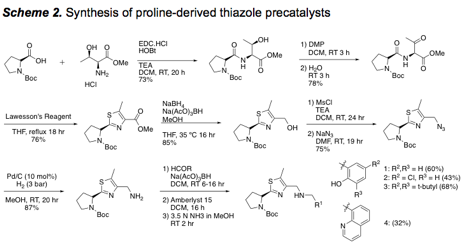
Continuing our efforts from the previous year, we continued to build a library of precatalysts designed to facilitate binding to Cu(I) for alkyne activation. Oxazole- and thiazole-based catalysts have been synthesized, and a focused library of 8 different thiazoles has been prepared (Schemes 1 and 2). The use of a sulfonic acid resin (Amberlyst 15) was effect for the 1-pot deprotection and purification (catch and release) of the final precatalysts.
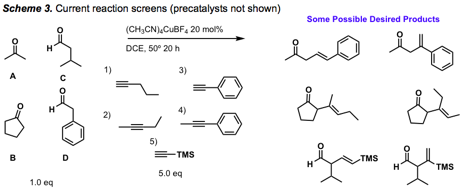
3. Reaction screens for Cu(I)-catalyzed additions of aldehydes/ketones to alkynes
Reaction screens are ongoing with a variety of aldehyde, ketone, and alkyne substrates (Scheme 3). Desired products from these reactions (>100) have not yet been identified, though byproducts from aldol reactions, catalyst alkylation, and alkyne dimerization have been partially characterized. The focus at the present time is on a study of additives, and also on obtaining x-ray structures of Cu(I) complexes with our precatalysts, which has not been successful thus far.
4. Next generation catalysts for use with group 10 metals
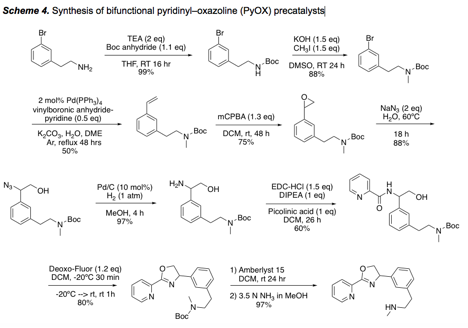
We have developed two synthetic routes to a new class of bifunctional pyridyl-oxazoline (PyOX) precatalysts designed for use with group 10 metals. Our latest route features a Pd-catalyzed vinylation, epoxidation, and epoxide opening with azide (Scheme 4). It could be rendered enantioselective with use of an asymmetric epoxidation reaction. The resulting catalysts will be screened in various ketone/alkyne coupling reactions.
Several related precatalysts (not shown) are in advanced stages of synthesis.
5. Career development
The ACS PRF has supported the stipends of two graduate students on this project, presently Jacob Porter and Eric Greve. Eric presented a poster on his early efforts on this project at the Chicago Organic Symposium in Oct. 2016, and Jacob was invited to attend and present a poster at the National ACS Division of Organic Chemistry Graduate Research Symposium in July 2017 in Portland, OR. The PI presented an overview of the bifunctional catalysis project at the National ACS Meeting in August 2016 in Washington, DC. One of the undergraduates whom we mentored from Milwaukee Area Technical College and who had worked on several aldol catalysts is now a graduate student in Chemistry at University of Wisconsin-Milwaukee.
In addition to the two full papers previously published on the aldol chemistry, a manuscript describing our early efforts at additions to alkyne with pi-acids, including DFT modeling and synthesis of novel catalysts, is in advanced stages. Support from the ACS has also enabled us to field two NSF grant applications in 2017 in order to continue our areas of investigation into dual and bifunctional catalysis.
Conclusions and current/future work
Our present focus is on the use of more rigid pincer catalysts, which we expect will provide more ready crystallographic and spectroscopic data than with our more flexible precatalysts. Preliminary DFT calculations with these systems are extremely promising. We expect to complete our studies with copper(I)-based bifunctional systems by the end of 2017, unless desired catalytic reactions can be characterized. We have also expanded our investigations to include dual catalytic systems, which are easier to access than bifunctional catalysts and are promising for certain substrate combinations.