Reports: ND155606-ND1: Synthesis of Cyclacenes: Strained Macrocyclic Hydrocarbons with Unique Bonding
Christopher J. Douglas, University of Minnesota
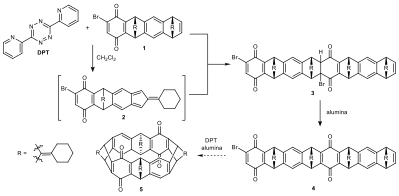
1. Attempts to Form a 8-Membered Macrocycle via Dimerization of Monoquinone 1
Attempts at macrocycle formation were first made using monoquinone 1. Specifically, Dr. Sarah Wegwerth targeted an [8]cyclacene precursor 5, which could be accessed through the dimerization of 1. Wegwerth proposed this reaction could work with slow addition of 3,6-Di-2-pyridyl-1,2,4,5-tetrazine (DPT) to monoquinone 1, thereby keeping the concentration of the intermediate isobenzofulvene 2 low relative to the dienophile 1. Before the macrocycle could be formed, a dehydrobromination would need to be performed to bring the two end groups into closer proximity. While only the isomer where the internal norbornadiene bridges are syn can give macrocycle, we envision that it would not need to be isolated as the anti isomer would likely polymerize upon reaction and be easily separated.
Scheme 1. Route to [8]cyclacene precursor 5.
As the crude mixture of the reaction towards 3 contained multiple stereo- and regio-isomers, it was subjected to a subsequent dehydrobromination via basic alumina. The resulted mixture showed an 1H NMR that contained key diagnostic proton signals of the desired dimer 4, which was also confirmed by LRMS experiments.
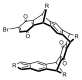
Macrocyclization attempts based on 4 were made, however, no evidence for the formation of macrocycle could be detected. We speculate this outcome is due to the possibility that a) the two ends are spatially too far apart to react in a Diels–Alder reaction manner (Figure 1) or b) the undesirable anti dimer was formed in appreciable quantities.
Figure 1. A 3-dimensional image of isobenzofulvene generated from 4. The cyclohexyl groups not shown for clarity.
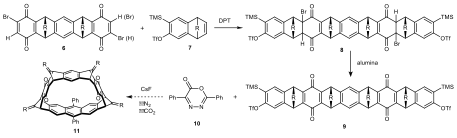
2. Efforts to Form a 10-Membered Macrocycle from Syn-bisquinone 6
In Scheme 2, we envision that a 10-membered macrocycle 11 could be built from 9 and 10 through a benzyne Diels−Alder and retro-Diels−Alder cascade. The Diels−Alder cycloaddition and dehydrobromination sequence was examined in our previous synthesis, which favored the formation of syn-isomers. In practice, we were able to add 7 to each side of 6 using the Diels−Alder cascade reaction. Formation of 8 was confirmed by 1H NMR, in which new signals around 2.7 ppm were observed, indicating that the quinones engaged in an exo Diels−Alder. Dehydrobromination of 8 was accomplished using basic alumina as indicated by the disappearance of the signals around 2.7 ppm. Although the 1H NMR of 9 isomers could not provide solid proof for the bridge configurations to be all syn, the few number of isomers (2 out of 6) observed indicated a stereoselective preference, in which the syn products should be favored due to steric effects. Encouraged by this result, we attempted to close the macrocycle. Treatment of bisaryne precursor 9 with CsF in the presence of diazapyrone 10 did not appear to yield the desired macrocycle. The bisaryne precursor 9 isomers were found partially consumed, leaving the major isomer unreacted. Multiple varations in reaction conditions in no reaction. We hypothesize that the all syn isomer of 9 is crowded around the TMS groups, making fluoride access difficult.
Scheme 2. Proposed synthesis of [10]cyclacene macrocyclic framework.
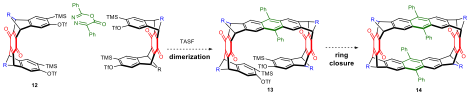
3. Accessing a 12-membered Macrocycle from Bisaryne Precursor 12
Graduate student Zhuoran Zhang attempted the synthesis of a [12]cyclacene derivative from bisaryne precursor 12 using similar approach illustrated in part 2. As shown in Scheme 3, Zhuoran proposed that the macrocyclization event could occur in a stepwise manner. In the first step, a dimer could be formed selectively by slow generation of benzyne. Applying the previous steric analysis, this intermolecular process should favor the formation of the syn isomer. In the second ring closing event, we envision it to be an easier process since the reaction sites have been placed in close proximity and no side reaction such as oligomerization should occur.
Scheme 3. Proposed synthesis of a 12-membered macrocycle 14.
In order to control the rate of benzyne formation, tris(dimethylamino)sulfonium difluorotrimethylsilicate (TASF), a soluable organic fluoride source was used to remove the TMS group. By varying the equivalence of bisbenzyne precursor 12 and diazapyrone 10, we found that the bisbenzyne precursor was efficiently consumed by the fluoride, however, the diazapyrone 10 was mostly recovered. This means that the benzyne Diels−Alder reaction did not occur and the benzyne species generated in situ decomposed under unknown pathways. This result indicated that the benzyne was either not stable or non-reactive.
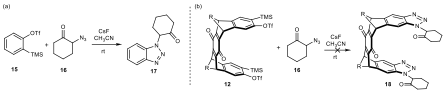
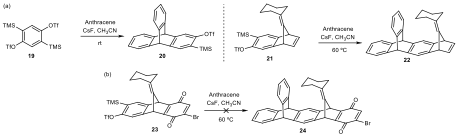
To test the utility of this benzyne intermediate, we performed a series of benzyne transformations based on precursors containing different segments. As shown in scheme 4, under similar conditions, benzyne precursor 15 were converted to the corresponding triazole. However, the bisbenzyne precursor 12 did not give the desired product. Moreover, shown in scheme 5, using anthracene as a diene also proved the poor reactivity of the benzyne precursor 24 containing the quinone moiety. These results strongly demonstrated that the quinone-containing benzyne precursor showed undesired reactivity. Subsequently, the route to the same 12-memberd macrocycle was revised as shown in scheme 6.
Scheme 4. Test of benzyne reactivity via click reactions.
Scheme 5. Test of benzyne reactivity via Diels−Alder reactions with anthracene.
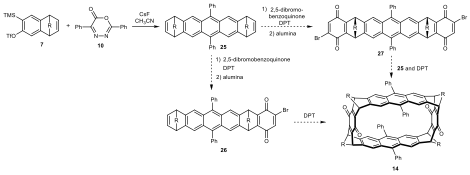
In this route, we plan to synthesize the anthracene derivative 25 using the benzyne Diels−Alder reaction, then apply tetrazine-initiated Diels−Alder cascade reactions as the ring closing method. We envision that the mono-quinone intermediate 26 could undergo a dimerization-cyclization process to form the 12-membered macrocycle 14. This route because does not involve any identification or purification of potential syn or anti isomers. Alternatively, the same macrocycle could be prepared via the reaction of syn-bisquinone 27 and anthracene derivative 25. In practice, we have synthesized the anthracene derivative 15 as a mixture of syn and anti isomers via a benzyne Diels−Alder reaction. Although the syntheses of more advanced intermediate are still underway, Wegwerth and Zhang have shown successful examples of installing the quinone moiety onto similar structures. Compared with our previous target in Scheme 1 and Figure 1, as the size of macrocycle 14 are larger by design, we expect this macrocyclization is possible since the two end functionalities would be closer together.
Scheme 6. Alternative synthetic routes to macrocycle 14.